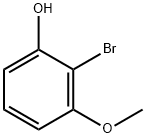
2-BROMO-3-METHOXYPHENOL synthesis
- Product Name:2-BROMO-3-METHOXYPHENOL
- CAS Number:135999-16-5
- Molecular formula:C7H7BrO2
- Molecular Weight:203.03
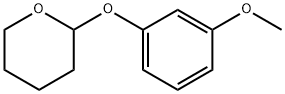
30778-86-0

135999-16-5
Tetrahydropyran-2-yl-3-methoxyphenyl ether (10 g, 48.1 mmol) was used as starting material and dissolved in 100 mL of tetrahydrofuran. Over 15 min, n-butyllithium (1.6 M hexane solution, 33 mL, 52.8 mmol) was added dropwise to this solution. After the dropwise addition, the reaction mixture was stirred at room temperature for 2.5 hours. Subsequently, the reaction mixture was cooled to 0 °C and 1,2-dibromoethane (4.6 mL, 53.2 mmol) was added dropwise. After completion of the dropwise addition, the reaction mixture was stirred at room temperature for about 14 hours. After the reaction was complete, the reaction mixture was diluted with 50 mL of 1N hydrochloric acid and stirred for 1 hour. The aqueous phase was extracted with three 100 mL portions of diethyl ether. The organic phases were combined and fully extracted with 5N sodium hydroxide solution. Combine the basic aqueous phase extracts and cool in an ice/water bath. The pH of the aqueous solution was adjusted to about 1 with 5N hydrochloric acid and then extracted with three 100 mL portions of diethyl ether. The ether extracts were combined, washed with saturated aqueous sodium chloride, dried over magnesium sulfate and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography using a gradient elution with hexane containing 0 to 10% ethyl acetate. The grades containing the target product 2-bromo-3-methoxyphenol were combined and concentrated under reduced pressure to give a residue of 2.91 g (30% yield), which was left to crystallize. Elemental Analysis (EA): C7H7BrO2 Calculated values: C, 41.41; H, 3.48. Measured values: C, 41.81; H, 3.46.

30778-86-0
1 suppliers
inquiry

135999-16-5
90 suppliers
$31.00/250mg
Yield: 30%
Reaction Conditions:
Stage #1:1-methoxy-3-tetrahydropyran-2-yloxybenzene with n-butyllithium in tetrahydrofuran;hexane at 20; for 2.75 h;
Stage #2: with ethylene dibromide in tetrahydrofuran;hexane at 0 - 20; for 14 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water for 1 h;
Steps:
VIII
33 mL (52.8 mMol) n-butyllithium (1.6 M in hexane)were added dropwise to a solution 10 gm (48.1 mMol) tetrahydropyran-2-yl 3-methoxyphenyl ether in 100 mL tetrahydrofuran over 15 minutes. After stirring for 2.5 hours at room temperature, the reaction mixture was cooled to 0° C. and then 4.6 mL (53.2 mMol) 1,2-dibromoethane were added dropwise. The reaction mixture was then allowed to stir at room temperature for about 14 hours. The reaction mixture was then diluted with 50 mL 1 N hydrochloric acid and was stirred for 1 hour. The aqueous phase was extracted with three 100 mL portions of diethyl ether. The organic phases were combined and extracted well with 5 N sodium hydroxide. These basic aqueous extracts were combined and cooled in an ice/water bath. The pH of this aqueous solution was adjusted to about 1 with 5 N hydrochloric acid and then extracted with three 100 mL portions of diethyl ether. These ether extracts were combined and washed with saturated aqueous sodium chloride, dried over magnesium sulfate and concentrated under reduced pressure. The resulting residue was subjected to silica gel chromatography, eluting with a gradient of hexane containing from 0 to 10% ethyl acetate. Fractions containing the desired compound were combined and concentrated under reduced pressure to provide 2.91 gm (30%) of a residue which crystallized upon standing.EA: Calculated for C7H7BrO2: Theory: C, 41.41; H, 3.48. Found: C, 41.81; H, 3.46.
References:
Eli Lilly and Company US7045545, 2006, B1 Location in patent:Page/Page column 22
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

135999-16-5
90 suppliers
$31.00/250mg
16932-45-9
150 suppliers
$36.00/1g

135999-16-5
90 suppliers
$31.00/250mg
150-19-6
360 suppliers
$6.00/5g

135999-16-5
90 suppliers
$31.00/250mg

150-19-6
360 suppliers
$6.00/5g
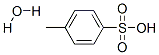
6192-52-5
582 suppliers
$1.70/25kg-r

30778-86-0
1 suppliers
inquiry

135999-16-5
90 suppliers
$31.00/250mg