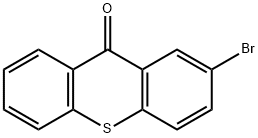
2-BROMO-10-THIAXANTHENONE synthesis
- Product Name:2-BROMO-10-THIAXANTHENONE
- CAS Number:20077-10-5
- Molecular formula:C13H7BrOS
- Molecular Weight:291.16
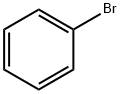
108-86-1
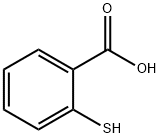
147-93-3

20077-10-5
To a 50 mL single-necked round-bottomed flask, 20 mL of concentrated sulfuric acid was added at room temperature, followed by the slow addition of 6 mL of bromobenzene and continuous stirring at room temperature. The reaction mixture was stirred for 30 minutes to form a white turbid liquid. Next, 1.0 g of thiosalicylic acid was added in batches over 30 minutes and the reaction continued to be stirred at room temperature for 20 to 24 hours. Subsequently, the reaction system was warmed to 100 °C and maintained for 2 to 3 hours. Upon completion of the reaction, it was cooled to room temperature and the reaction solution was carefully poured into ice water. The precipitated solid product was collected by diafiltration. The resulting solid was suspended in 20% aqueous sodium hydroxide solution, stirred for 2 hours and then diafiltered again. Finally, the filter cake was washed with water to neutrality to give the yellow solid product 2-bromo-9H-thioxanthen-9-one in 80.3% yield.
Yield:20077-10-5 87.9%
Reaction Conditions:
Stage #1: phenyl bromidewith sulfuric acid in dichloromethane; for 0.5 h;
Stage #2: Thiosalicylic acid in dichloromethane; for 2 h;
Steps:
2.2.1.1. Synthesis of 2-bromo-9H-thioxanthen-9-one (X1)
Cyclization[14]. Bromobenzene (5 mmol, 0.78 g) was added to sulfuric acid(10 ml) and methylene chloride (100 ml), followed by stirring themixture for 30 min. After adding thiosalicylic acid (5 mmol, 0.77g), themixture was stirred for 2 h. When the reaction was completed, the solutionwas cooled to room temperature and water was added. Thegenerated crystals were filtered under reduced pressure, followed by thewashing with 10% aqueous NaOH solution (200 ml). These crystals wererecrystallized using ethanol, affording the final product in 87.9% yield.1H NMR (400 MHz, DMSO-d6): 7.41 (d, J = 8.1 Hz, 2H, ArH), 7.54 (s,2H, ArH), 7.66 (d, J = 7.9 Hz, 1H, ArH), 8.10 (d, J = 1.6 Hz, 2H, ArH);GC-MS, 292(M+); Elemental analysis Found C: 53.62, H: 2.43, Br: 27.42,O: 5.51, S: 11.01 calculated for C13H7BrOS C: 53.63, H: 2.42, Br: 27.44,O: 5.49, S: 11.01.
References:
Kang;Lee;Choi [Dyes and Pigments,2022,vol. 206,art. no. 110594]
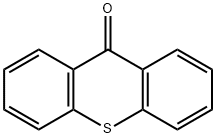
492-22-8
198 suppliers
$6.00/1g

20077-10-5
47 suppliers
inquiry
![Benzoic acid, 2-[(4-bromophenyl)thio]-](/CAS/20210305/GIF/13420-76-3.gif)
13420-76-3
1 suppliers
inquiry

20077-10-5
47 suppliers
inquiry

88-67-5
564 suppliers
$5.00/5g

20077-10-5
47 suppliers
inquiry
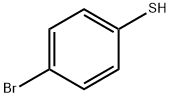
106-53-6
291 suppliers
$12.00/1g

20077-10-5
47 suppliers
inquiry