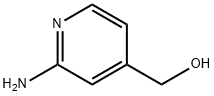
(2-AMINO-PYRIDIN-4-YL)-METHANOL synthesis
- Product Name:(2-AMINO-PYRIDIN-4-YL)-METHANOL
- CAS Number:105250-17-7
- Molecular formula:C6H8N2O
- Molecular Weight:124.14

6937-03-7

105250-17-7
General procedure for the synthesis of 2-amino-4-pyridinemethanol from methyl 2-aminoisonicotinate: 26 g of lithium aluminum hydride was dissolved in 800 mL of anhydrous tetrahydrofuran (THF). Under stirring conditions, 103 g of 2-aminoisonicotinic acid methyl ester dissolved in 600 mL of anhydrous THF was slowly added, and the resulting slurry was heated to reflux for 3 hours. Upon completion of the reaction, the reaction was quenched by careful addition of cooling water, and the precipitate was collected by filtration and washed with 300 mL of THF. The filtrates were combined and concentrated under reduced pressure and the residue was recrystallized from benzene. The product was obtained as 61 g in 73% yield with a melting point of 80-81.5°C. The product was analyzed by 1H NMR (DMSO-d6, δ, ppm): 4.36 (s, 2H), 5.19 (s, 1H), 5.78 (s, 2H), 6.40 (d, 1H), 6.46 (s, 1H), 7.81 (d, 1H).13C NMR (DMSO- d6, δ, ppm): 62.3, 105.2, 110.3, 147.7, 152.7, 160.3. Elemental analysis results (%): C 57.63, H 6.32, N 22.68; theoretical values (C6H8N2O): C 58.05, H 6.40, N 22.56.

6937-03-7
213 suppliers
$8.00/1g

105250-17-7
223 suppliers
$7.00/250mg
Yield:105250-17-7 73%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran for 3 h;Reflux;
Steps:
(2-Aminopyridin-4-yl)methanol (1).
26 g of lithiumaluminum hydride was dissolved in 800 mL ofanhydrous THF. A solution of 103 g of methyl ether of2-aminoisonicotinate in 600 mL of anhydrous THFwas added at stirring and the formed slurry was boiledfor 3 h. After cooling water was carefully added, theprecipitate was filtered off and washed with 300 mL ofTHF. The combined filtrates were evaporated, theresidue was crystallized from benzene. Yield 61 g(73%), mp 80-81.5°. 1H NMR spectrum (DMSO-d6),δ, ppm: 4.36 s (2), 5.19 s (1), 5.78 s (2), 6.40 d(1), 6.46 s (1), 7.81 d (1). 13 NMR spectrum(DMSO-d6), δ, ppm: 62.3, 105.2, 110.3, 147.7, 152.7,160.3. Found, %: 57.63; 6.32; N 22.68. C6H8N2O.Calculated, %: C 58.05; H 6.4; N 22.56.
References:
Lifshits;Ostapchuk;Brel [Russian Journal of Organic Chemistry,2015,vol. 51,# 5,p. 744 - 745][Zh. Org. Khim.,2015,vol. 51,# 5,p. 759 - 760,2]
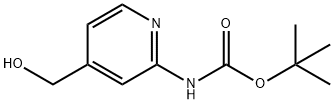
304873-62-9
48 suppliers
$95.00/250mg

105250-17-7
223 suppliers
$7.00/250mg
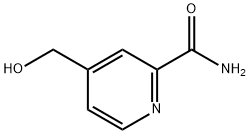
119646-48-9
17 suppliers
inquiry

105250-17-7
223 suppliers
$7.00/250mg
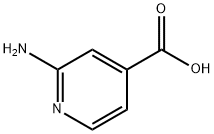
13362-28-2
313 suppliers
$6.00/1g

105250-17-7
223 suppliers
$7.00/250mg
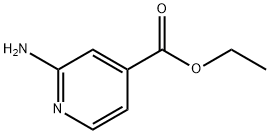
13362-30-6
138 suppliers
$6.00/250mg

105250-17-7
223 suppliers
$7.00/250mg