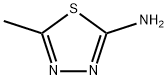
2-Amino-5-methyl-1,3,4-thiadiazole synthesis
- Product Name:2-Amino-5-methyl-1,3,4-thiadiazole
- CAS Number:108-33-8
- Molecular formula:C3H5N3S
- Molecular Weight:115.16
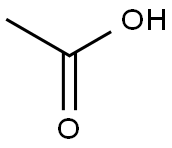
64-19-7
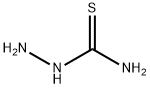
79-19-6

108-33-8
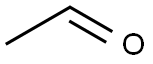
1) 0.05 mol of aminothiourea was added to a dry mortar, followed by 0.055 mol of glacial acetic acid, 0.055 mol of silica, and 0.25 mol of phosphorus trichloride, and ground for 10 min at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) using a solvent mixture of ethyl acetate and petroleum ether in the ratio of 1:3 by volume as the unfolding agent until the point of raw material aminothiourea disappeared, indicating that the reaction was complete. The crude product was obtained after standing for 30 min.2) The crude product was transferred to a beaker and the pH was adjusted to 8 by adding 5% sodium carbonate solution.The mixture was withdrawn and filtered and the filter cake was dissolved with N,N-dimethylformamide (DMF) to remove silica gel. The filtrate was concentrated under reduced pressure, and after removing the solvent, washed with water and filtered by suction to afford 2-amino-5-methyl-1,3,4-thiadiazole in 95.2% yield.
Yield:108-33-8 96.3%
Reaction Conditions:
with choline chloride;urea at 80; for 1 h;
Steps:
1.1-1.3 Example 1Preparation of 2-amino-5-methyl-1,3,4-thiadiazole
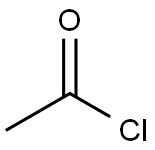
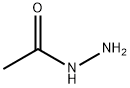
In the first step, 1 mol of choline chloride and 2 mol of urea are added to the reaction vessel.Stir at 80 ° C until fully dissolved to obtain a eutectic solvent;In the second step, after cooling the reaction system to room temperature, 1 mol of acetic acid and 1.2 mol were added.Thiosemicarbazone, slowly heating, refluxing at 80 ° C for 1h,TLC monitors the end of the reaction;The third step is to cool the reaction mixture to room temperature.Then adjust the pH to 8-9 with 10% ammonia solution under ice-bath cooling.The solid precipitated out, filtered with suction, the filter cake was washed with ice water, and then recrystallized.Obtained 2-amino-5-methyl-1,3,4-thiadiazole, yield 96.3% the filtrate was recovered to obtain a eutectic solvent.
References:
CN110724115,2020,A Location in patent:Paragraph 0038-0044