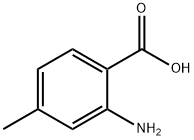
2-Amino-4-methylbenzoic acid synthesis
- Product Name:2-Amino-4-methylbenzoic acid
- CAS Number:2305-36-4
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
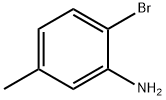
53078-85-6

2305-36-4
The general procedure for the synthesis of 2-amino-4-methylbenzoic acid from 2-bromo-5-methylaniline was as follows: 2-bromo-5-methylaniline (10.0 g, 53.7 mmol) was dissolved in anhydrous ethyl ether (500 mL) under protection of nitrogen atmosphere, cooled to -78 °C and stirred. Tert-butyl lithium (127 mL, 1.7 M solution, 214.8 mmol) in pentane was added slowly over 15 minutes, keeping the reaction temperature from exceeding -65 °C. After addition, stirring was continued at -78°C for 1.5 hours. Subsequently, the reaction was quenched with excess crushed dry ice (solid CO2). After complete evaporation of the dry ice, water was added to the reaction mixture and the organic layer was separated and discarded. The aqueous layer was acidified with 1N hydrochloric acid and then extracted with two parts of ethyl acetate. The organic extracts were combined, dried with anhydrous magnesium sulfate, filtered and concentrated to give the target product 2-amino-4-methylbenzoic acid (4.8 g, 59% yield) as a tan crystalline solid. Mass spectrum (chemical ionization): 152 (M+H).
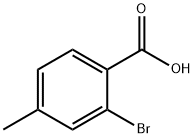
7697-27-0
211 suppliers
$6.00/1g

2305-36-4
200 suppliers
$6.00/1g
Yield:2305-36-4 87%
Reaction Conditions:
with ammonium hydroxide;L-2-O-methyl-chiro-inositol;copper(II) acetate monohydrate in 1-methyl-pyrrolidin-2-one at 110; for 12 h;
Steps:
Synthesis of 5a–z, 5aa; General Procedure C
General procedure: To a sealed reaction vessel were added ammonia (aq, 25percent) (1.8 mL, 12.8 mmol),Cu(OAc)2·H2O (0.13 mmol), QCT (0.26 mmol) in NMP (1.8 mL) and aryl halides(1.28 mmol). The resulting mixture was heated in an oil bath with appropriatetemperature under rapid stirring for the indicated time. After complete consumption ofthe aryl halide as monitored by TLC, the reaction vessel was cooled to r.t. It was openedto air, and the reaction mixture was extracted with ethyl acetate (3×10 mL), and theorganic layer was washed with water (2×10 mL) and once with brine (10 mL), driedwith magnesium sulfate and concentrated in vacuo. The product was purified bycolumn chromatography on silica gel.
References:
Chen, Guoliang;Chen, Yuanguang;Du, Fangyu;Fu, Yang;Wu, Ying;Zhou, Qifan [Synlett,2019,vol. 30,# 19,p. 2161 - 2168] Location in patent:supporting information
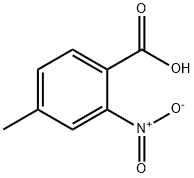
27329-27-7
103 suppliers
$7.00/250mg

2305-36-4
200 suppliers
$6.00/1g

53078-85-6
201 suppliers
$6.00/1g

2305-36-4
200 suppliers
$6.00/1g
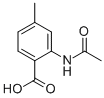
81115-52-8
4 suppliers
inquiry
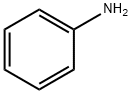
62-53-3
689 suppliers
$10.00/1g

103-84-4
357 suppliers
$12.00/100g

2305-36-4
200 suppliers
$6.00/1g
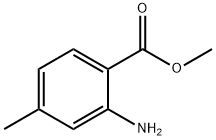
18595-17-0
98 suppliers
$9.00/100mg

2305-36-4
200 suppliers
$6.00/1g