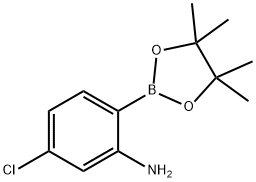
2-AMINO-4-CHLOROPHENYL BORONIC ACID PINACOL ESTER synthesis
- Product Name:2-AMINO-4-CHLOROPHENYL BORONIC ACID PINACOL ESTER
- CAS Number:863578-21-6
- Molecular formula:C12H17BClNO2
- Molecular Weight:253.53
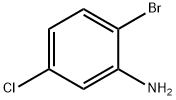
823-57-4
25015-63-8

863578-21-6
Step 1: Preparation of 5-chloro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline To a solution of 2-bromo-5-chloroaniline (20.0 g, 96.9 mmol) in 1,4-dioxane (200 ml) was sequentially added triethylamine (54.0 ml, 487 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride dichloromethane complex (3.96 g, 4.84 mmol) under nitrogen protection. Subsequently, 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (42.3 ml, 290 mmol) was slowly added dropwise. The reaction mixture was stirred at 100 °C for 10.5 hours. After completion of the reaction, the mixture was cooled to room temperature and filtered through diatomaceous earth. The filtrate was quenched by dropwise addition of methanol (25 ml) at 0°C. The solvent was removed by concentration under reduced pressure and the resulting crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 20:1) to afford the target product 5-chloro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (16.5 g, 65% yield). 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 7.28 (1H, d, J=6.0 Hz), 6.59 (1H, d, J=1.2 Hz), 6.43 (1H, dd, J=6.3,1.5 Hz), 5.70 (2H, s), 1.24 (12H, s).

823-57-4
180 suppliers
$6.00/1g

863578-21-6
76 suppliers
$28.00/100mg
Yield: 96%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate;bis(pinacol)diborane in dimethyl sulfoxide at 85;Inert atmosphere;
Steps:
14.3 Step 3: Synthesis of 5-chloro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
To a round bottomed flask was added PdCl2(dppf)-CH2Cl2 adduct (1.347 g, 1.650 mmol), KOAc (6.48 g, 66.0 mmol), bis(pinacolato)diboron (12.57 g, 49.5 mmol), 2- bromo-5-chloroaniline (6.81 g, 33 mmol), and DMSO (100 mL). The solution was evacuated/backfilled with N2 (3x) before heating to 85 °C overnight. The solution was cooled to room temperature and diluted with MTBE and water. The solution was filtered and the layers were separated. The aqueous phase was extracted with MTBE and the combined organics layer was washed with water, brine, dried over Na2504, filtered, and concentrated. The crude residue was purified via Biotage to afford 5-chloro-2-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)aniline (8.06 g, 31.8 mmol, 96% yield). m/z (ESI) 254 [M+H]+
References:
CONSTELLATION PHARMACEUTICALS, INC.;ALBRECHT, Brian, K.;GEHLING, Victor, S.;TAYLOR, Alexander, M.;VASWANI, Rishi, G. WO2013/184878, 2013, A1 Location in patent:Paragraph 00183; 00184

823-57-4
180 suppliers
$6.00/1g

25015-63-8
216 suppliers
$22.39/5G

863578-21-6
76 suppliers
$28.00/100mg

823-57-4
180 suppliers
$6.00/1g
73183-34-3
583 suppliers
$6.00/5g

863578-21-6
76 suppliers
$28.00/100mg
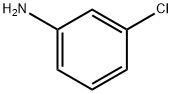
108-42-9
471 suppliers
$10.00/5g

863578-21-6
76 suppliers
$28.00/100mg

108-42-9
471 suppliers
$10.00/5g

73183-34-3
583 suppliers
$6.00/5g

25015-63-8
216 suppliers
$22.39/5G

863578-21-6
76 suppliers
$28.00/100mg
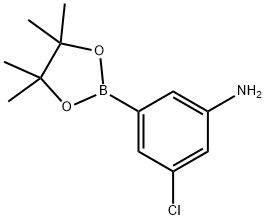
1269233-11-5
13 suppliers
inquiry