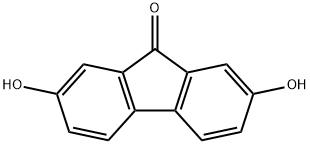
2,7-Dihydroxy-9-fluorenone synthesis
- Product Name:2,7-Dihydroxy-9-fluorenone
- CAS Number:42523-29-5
- Molecular formula:C13H8O3
- Molecular Weight:212.2
![[1,1'-Biphenyl]-2-carboxylic acid, 4,4'-dihydroxy-, methyl ester](/CAS/20200611/GIF/765944-63-6.gif)
765944-63-6

42523-29-5
Methyl 4,4'-dihydroxy-[1,1'-biphenyl]-2-carboxylate (50 g, 0.20 mol) was used as a raw material and mixed with ZnCl2 (50 g, 0.37 mol) and polyphosphoric acid (PPA, 33 mL). The reaction mixture was stirred at 110-120 °C for 2 hours. Upon completion of the reaction, it was cooled to room temperature and water (500 mL) was slowly added to precipitate the product. The precipitate was collected by filtration and dried to give 40 g of reddish brown crystalline powder (2,7-dihydroxy-9-fluorenone). The yield was 95%; melting point 336-337°C (literature value 338°C [24]); IR (KBr) ν (cm^-1): 3388.9, 3360.6 (OH), 1700.7 (C=O); ESI-MS (m/z): 211.0 [M+H]^+, molecular formula C13H8O3 (molecular weight 212.0); 1H- NMR (300MHz, DMSO-d6) δ (ppm): 9.89 (s, 2H), 7.37 (d, J=4.8Hz, 2H), 6.90 (dd, J=4.9,2.4Hz, 2H), 6.86 (d, J=2.4Hz, 2H).
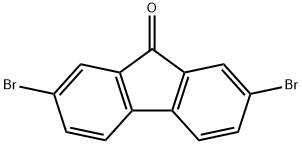
14348-75-5
314 suppliers
$14.00/1g

42523-29-5
263 suppliers
$6.00/1g
Yield:42523-29-5 865 mg
Reaction Conditions:
with lithium hydroxide monohydrate;N1,N2-bis(4-hydroxy-2,6-dimethylphenyl)oxalamide;copper(II) acetylacetonate in water;dimethyl sulfoxide at 120; for 4 h;
Steps:
4 Synthesis of 2,7-dihydroxyfluoren-9-one using bis (2,4-pentanedionato) copper (II)
2,7-dibromofluoren-9-one (manufactured by Tokyo Chemical Industry Co., Ltd., 1.35 g, 4.0 mmol),Lithium hydroxide monohydrate (704 mg, 16.8 mmol),Bis (2,4-pentanedionato) copper (II) (53.6 mg, 0.20 mmol),A mixture of N, N'-bis (2,6-dimethyl-4-hydroxyphenyl) oxamide (65.0 mg, 0.20 mmol) and DMSO / water (9.6 mL / 2.4 mL) at 120 ° C. for 4 hours Heated and stirred. After cooling to room temperature, the reaction mixture is diluted with waterFurthermore, 3 M hydrochloric acid is added to adjust the pH to acidic,The resulting red solid was collected by filtration and vacuum dried (100 ° C., overnight).The target 2,7-dihydroxyfluoren-9-one was obtained as a red solid (865 mg, quantitative, HPLC purity: 85.6%).
References:
JP2019/77634,2019,A Location in patent:Paragraph 0106-0108
![[1,1'-Biphenyl]-2-carboxylic acid, 4,4'-dihydroxy-, methyl ester](/CAS/20200611/GIF/765944-63-6.gif)
765944-63-6
0 suppliers
inquiry

42523-29-5
263 suppliers
$6.00/1g
202865-84-7
33 suppliers
$67.00/250mg

100-83-4
654 suppliers
$5.00/10g

42523-29-5
263 suppliers
$6.00/1g
53197-57-2
38 suppliers
$378.00/1g

42523-29-5
263 suppliers
$6.00/1g

42523-28-4
0 suppliers
inquiry

42523-29-5
263 suppliers
$6.00/1g