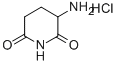
2,6-Dioxopiperidine-3-ammonium chloride synthesis
- Product Name:2,6-Dioxopiperidine-3-ammonium chloride
- CAS Number:24666-56-6
- Molecular formula:C5H9ClN2O2
- Molecular Weight:164.5902

24666-55-5

24666-56-6
General procedure for the synthesis of 3-aminopiperidine-2,6-dione hydrochloride from benzyl (2,6-dioxopiperidin-3-yl)carbamate: Benzyl (2,6-dioxopiperidin-3-yl)carbamate (4.00 g, 15.0 mmol) was dissolved in methanol (200 mL) and 2N HCl solution (15 mL) was added. Subsequently, 5% Pd-C catalyst (100 mg) was added and the hydrogenation reaction was carried out at 60 psi hydrogen pressure for 4 hours. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated to dryness to afford 3-aminopiperidine-2,6-dione hydrochloride as a white solid (2.61 g, 100% yield) with a melting point of 245 °C (decomposition, ignition temperature 235 °C).1H NMR (400 MHz, DMSO-D6) δ ppm: 11.22 (br s, 1H), 8.68 (br s, 3H), 4.20 (dd, J = 13.0, 5.3 Hz, 1H), 2.77-2.65 (m, 1H), 2.64-2.56 (m, 1H), 2.27-2.19 (m, 1H), 2.09-1.97 (m, 1H).

24666-55-5
85 suppliers
$37.00/100mg

24666-56-6
432 suppliers
$8.00/5g
Yield:24666-56-6 100%
Reaction Conditions:
with hydrogenchloride;hydrogen;5% palladium over charcoal in methanol;water; under 3102.97 Torr; for 4 h;
Steps:
1
A solution of 15 (4.0Og, 0.015 mol) in methanol (200 ml) and 2N HCl (15 ml) was hydrogenated over 5% Pd-C (100 mg) at 60 psi for 4 h. The catalyst was filtered off and the filtrate concentrated to dryness to give the title compound 16 as a white solid (2.61 g, 100%), mp 245 0C (dec) (lit. 235 0C (dec)). 1H NMR (400 MHz, DMSO-D6) δ ppm 11.22 (br s? IH), 8.68 (br s, 3H), 4.20 (dd, J=13.0, 5.3 Hz, IH), 2.77-2.65 (m, IH), 2.64- 2.56 (m, IH), 2.27-2.19 (m, IH)5 2.09-1.97 (m, IH).
References:
WO2008/7979,2008,A1 Location in patent:Page/Page column 13
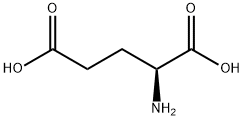
617-65-2
274 suppliers
$6.00/25g

24666-56-6
432 suppliers
$8.00/5g
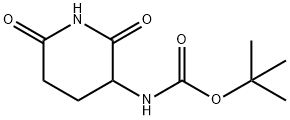
31140-42-8
131 suppliers
$8.00/250mg

24666-56-6
432 suppliers
$8.00/5g

2650-64-8
363 suppliers
$10.00/5g

24666-56-6
432 suppliers
$8.00/5g

6398-06-7
30 suppliers
$89.59/1G

24666-56-6
432 suppliers
$8.00/5g