
2,6-DICHLOROBENZENESULFONYL CHLORIDE synthesis
- Product Name:2,6-DICHLOROBENZENESULFONYL CHLORIDE
- CAS Number:6579-54-0
- Molecular formula:C6H3Cl3O2S
- Molecular Weight:245.51
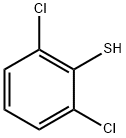
24966-39-0

6579-54-0
a) To 200 mL of mixed solvent (acetic acid/water/dichloromethane, 3/1/4 by volume) were added 2,6-dichlorothiophenol (10.0 g, 55.8 mmol), N-chlorosuccinimide (37.28 g, 279 mmol) and potassium acetate (2.29 g, 27.9 mmol). The reaction mixture was stirred at 0 °C and subsequently heated to room temperature. After completion of the reaction, the mixture was diluted with 200 mL of dichloromethane and washed three times with 100 mL of water. The organic layer was dried with anhydrous sodium sulfate and concentrated to give 2,6-dichlorobenzenesulfonyl chloride (11 g, 80% yield). The product was confirmed by NMR hydrogen spectroscopy (CDCl3): δ 7.57 (d, 2H), 7.47 (t, 1H).

24966-39-0
177 suppliers
$20.00/5g

6579-54-0
168 suppliers
$7.00/1g
Yield: 80%
Reaction Conditions:
with N-chloro-succinimide;potassium acetate in dichloromethane
Steps:
a a)
a) 2,6-dichlorobenzenesulfonyl chloride Into a mixture of 200 milliliters (hereinafter "mL") of acetic acid, water and dichloromethane (3/1/4, v/v/v), 2,6 dichlorobenzenethiol (10.0 grams (hereinafter "g"), 55.8 millimoles (hereinafter "mmol"), N-chlorosuccinimide (37.28 g, 279 mmol) and potassium acetate (2.29 g, 27.9 mmol) were added. The resulting mixture was stirred at 0° C., then warmed to room temperature overnight. The mixture was then diluted with 200 mL of dichloromethane, and washed with water (100 mL*3). The organic layer was dried (Na2SO4) and concentrated to give the desired product (11 g, 80%). 1H NMR (CDCl3): δ7.57 (d, 2H), 7.47 (t, 1H).
References:
Widdowson, Katherine Louisa;Jin, Qi US2003/78250, 2003, A1
7446-09-5
143 suppliers
$220.00/454g
608-31-1
320 suppliers
$12.00/25g

6579-54-0
168 suppliers
$7.00/1g