
2,5,6-tetrachloropyridine synthesis
- Product Name:2,5,6-tetrachloropyridine
- CAS Number:54718-39-7
- Molecular formula:C6H2Cl3NO2
- Molecular Weight:226.44
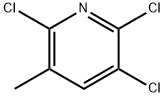
58584-95-5

54718-39-7
General procedure for the synthesis of 2,5,6-trichloronicotinic acid from 2,3,6-trichloro-5-methylpyridine: 2,3,6-trichloro-5-methylpyridine (18.8 g, 60.0 mmol) was suspended in water (400 ml) and heated to 100 °C. Subsequently, potassium permanganate (KMnO4, 28.5 g, 180.2 mmol) was added in batches over 12 hours. The reaction mixture was stirred continuously at 100 °C for 2 days, during which additional potassium permanganate (10 g) was added. After confirming the complete consumption of raw materials by TLC monitoring, the reaction mixture was filtered while hot and washed with hot water (2 x 75 ml). The combined filtrates were cooled to room temperature and extracted with ethyl acetate (EtOAc, 3 x 100 ml). The aqueous phase was concentrated to about 50 ml, cooled to 0 °C and the pH was adjusted to 1-2 with 6.0 M hydrochloric acid.The precipitated solid was collected by filtration, washed with cold water and dried to afford the target product 2,5,6-trichloronicotinic acid (2.5 g, 18% yield), which was used in subsequent reactions without further purification.

58584-95-5
29 suppliers
inquiry

54718-39-7
156 suppliers
$11.00/250mg
Yield:54718-39-7 18%
Reaction Conditions:
Stage #1: 2,5,6-trichloro-3-methylpyridinewith potassium permanganate in water at 100; for 60 h;
Stage #2: with hydrogenchloride in water at 0; pH=1 - 2;
Steps:
47
2,5,6-Trichloronicotinic acid A suspension of 2,3,6-trichloro-5-methylpyridine (Method 48, 11.8 g, 60.0 mmol) in water (400 ml) was heated to 100 0C. Portionwise, KMnO4 (28.5 g, 180.2 mmol) was then added over 12 hours. The reaction was then allowed to stir for 2 days at 100 0C, over which time an additional 1O g of KMnO4 was added portionwise. When no starting material EPO
References:
WO2006/82392,2006,A1 Location in patent:Page/Page column 109-110

29553-51-3
42 suppliers
$47.00/100mg

54718-39-7
156 suppliers
$11.00/250mg

58584-80-8
3 suppliers
inquiry

54718-39-7
156 suppliers
$11.00/250mg

52465-59-5
18 suppliers
inquiry

54718-39-7
156 suppliers
$11.00/250mg
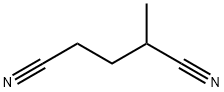
4553-62-2
120 suppliers
$6.00/5g

54718-39-7
156 suppliers
$11.00/250mg