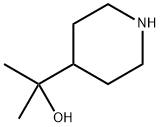
2-(4-PIPERIDYL)-2-PROPANOL synthesis
- Product Name:2-(4-PIPERIDYL)-2-PROPANOL
- CAS Number:22990-34-7
- Molecular formula:C8H17NO
- Molecular Weight:143.23

160809-39-2

22990-34-7
Benzyl 4-(1-hydroxy-1-methylethyl)piperidine-1-carboxylate (1.786 g, 6.44 mmol) was used as starting material and dissolved in methanol (100 ml) under nitrogen protection. A 10% palladium-carbon catalyst (50% wet, 1.37 g) was added, followed by replacement of the gas in the reaction system with hydrogen at atmospheric pressure. The reaction mixture was stirred at room temperature for overnight reaction. Upon completion of the reaction, the hydrogen in the reaction system was replaced with nitrogen, then the catalyst was removed by filtration and washed with methanol. The filtrate and washings were combined and the solvent was removed by distillation. Finally, the residue was dried under reduced pressure to afford the target product 2-(4-piperidinyl)-2-propanol (922 mg, 6.44 mmol, quantitative yield) as light gray crystals. The product was characterized by 1H-NMR (CDCl3), δ (ppm): 1.18 (6H, s), 1.26-1.42 (3H, m), 1.74-1.80 (2H, m), 2.57-2.64 (2H, m), 3.14-3.22 (2H, m), 3.48 (1H, s).

160809-39-2
0 suppliers
inquiry

22990-34-7
125 suppliers
$22.00/1g
Yield: 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol under 760.051 Torr;
Steps:
42-3 Production example 42-3; 4-(1-Hydroxy-1-methylethyl)piperidine
Benzyl 4-(1-hydroxy-1-methylethyl)piperidine-1-carboxylate (1.786 g, 6.44 mmol) synthesised in Production example 42-2 was dissolved in methanol (100 ml) under nitrogen atmosphere; 10% palladium on carbon (50% wet, 1.37 g) was added; the reaction system was purged with hydrogen at atmospheric pressure; and the reaction mixture was stirred overnight. After the reaction system was purged with nitrogen, the catalyst was filtered out, and washed with methanol; the solvent, together with the filtrate and the washing solution, was distilled off; and the residue was dried under reduced pressure to yield the title compound (922 mg, 6.44 mmol, quantitative) as pale gray crystals. 1H-NMR Spectrum (CDCl3) δ (ppm): 1.18 (6H, s), 1.26-1.42 (3H, m), 1.74-1.80 (2H, m), 2.57-2.64 (2H, m), 3.14-3.22 (2H, m), 3.48 (1H, s).
References:
Eisai Co., Ltd. EP1522540, 2005, A1 Location in patent:Page/Page column 64
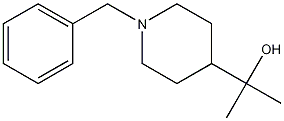
299428-04-9
33 suppliers
$18.00/250mg

22990-34-7
125 suppliers
$22.00/1g
1126-09-6
477 suppliers
$15.40/25g

22990-34-7
125 suppliers
$22.00/1g
2971-79-1
273 suppliers
$10.00/10g

22990-34-7
125 suppliers
$22.00/1g
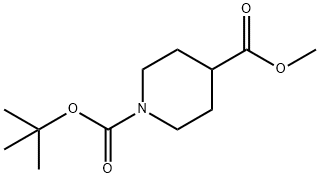
124443-68-1
254 suppliers
$6.00/5g

22990-34-7
125 suppliers
$22.00/1g
