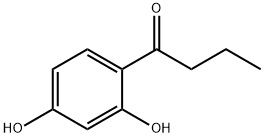
2',4'-dihydroxybutyrophenone synthesis
- Product Name:2',4'-dihydroxybutyrophenone
- CAS Number:4390-92-5
- Molecular formula:C10H12O3
- Molecular Weight:180.2
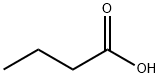
107-92-6
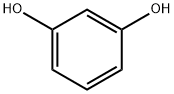
108-46-3

4390-92-5
To a 2.0 L four-neck flask was added 165.0 g of resorcinol (1.5 mol) followed by 1.0 L of toluene as a solvent. Under stirring conditions, 158.6 g (1.8 mol) of n-butyric acid was slowly added, followed by 245.3 g (1.8 mol) of zinc chloride as a catalyst. The reaction mixture was heated to reflux while the water generated during the reaction was separated by means of a water separator. The reaction process was monitored by gas chromatography (GC) until the reaction was completed. Upon completion of the reaction, the zinc catalyst was first removed and then the organic phase was washed sequentially with water and saturated sodium bicarbonate solution for purification. Finally, 241.5 g of 4-butyrylresorcinol was obtained by concentrating the organic phase in 89.3% yield.

107-92-6
598 suppliers
$9.00/5g

108-46-3
795 suppliers
$10.00/10g

4390-92-5
88 suppliers
$38.00/100mg
Yield: 99%
Reaction Conditions:
with boron trifluoride diethyl etherate in chlorobenzene at 80;Friedel-Crafts Acylation;
Steps:
2.3.1. General method for the synthesis of the precursor ketones
General procedure: A mixture of the appropriate carboxylic acid (5.44 mmol) and resorcinol (1.36 mmol) in C6H5Cl (0.6 mL) was treated with BF3.Et2O (5.44 mmol). The reaction was stirred at 80 °C until complete consumption of resorcinol was assessed by TLC. The reaction mixture was treated with brine (10 mL) and the products were extracted with EtOAc (3 × 10 mL). The organic layers were combined, dried over MgSO4 and concentrated under reduced pressure. The residues were purified by column chromatography, eluting with mixtures of hexanes and EtOAc
References:
Cortés, Iván;di Liberto, Melina G.;Kaufman, Teodoro S.;Derita, Marcos G.;Bracca, Andrea B.J. [Food Chemistry,2020,vol. 321,art. no. 126701]
4346-18-3
41 suppliers
inquiry

108-46-3
795 suppliers
$10.00/10g

4390-92-5
88 suppliers
$38.00/100mg
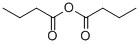
106-31-0
235 suppliers
$10.00/5g

108-46-3
795 suppliers
$10.00/10g

4390-92-5
88 suppliers
$38.00/100mg

141-75-3
359 suppliers
$12.32/25G

108-46-3
795 suppliers
$10.00/10g

4390-92-5
88 suppliers
$38.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4390-92-5
88 suppliers
$38.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry