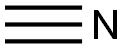2 4-DIHYDROXY-6-METHYLBENZALDEHYDE synthesis
- Product Name:2 4-DIHYDROXY-6-METHYLBENZALDEHYDE
- CAS Number:487-69-4
- Molecular formula:C8H8O3
- Molecular Weight:152.15
504-15-4
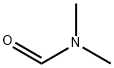
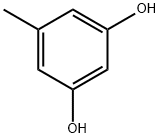
68-12-2

487-69-4
5-Methylbenzene-1,3-diol (25.0 g, 0.2 mol) was dissolved in N,N-dimethylformamide (100 mL) at 0 °C and slowly added dropwise to a pre-cooled mixture of POCl3 (44.0 g, 0.3 mol) and N,N-dimethylformamide (200 mL). After the dropwise addition was completed, the reaction mixture was stirred at room temperature for 1 hour. Subsequently, the reaction mixture was slowly poured into ice water and the solid product was precipitated. The solid was collected by filtration and washed three times with distilled water to remove residual acid and solvent. Finally, the product was dried to afford 2,4-dihydroxy-6-methylbenzaldehyde (21.0 g, 82.0% yield), which could be used in subsequent reactions without further purification.

504-15-4
404 suppliers
$7.00/100mg
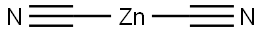
557-21-1
105 suppliers
$12.00/5g

487-69-4
78 suppliers
$24.00/250mg
Yield: 76%
Reaction Conditions:
Stage #1:orcinol;zinc(II) cyanide with hydrogenchloride in diethyl ether for 2 h;Inert atmosphere;
Stage #2: with water at 100;
Steps:
3.1 Example 3 - Synthesis of Colletochlorin B
Step 1: Compound (i) to compound (2) Orcinol (5g, 40mmol) and Zn(CN)2 (7-ig, 6ommol) were placed into a 3 necked flask with mechanical stirrer under N2. 50ml of Ether was added, and the reaction was saturated with HC1 gas. After 2 hours, the Ether was decanted off and 50mls of water added to the reaction mixture. This was heated to ioo°C where the product crashed out of solution. The crude product was collected via buchner filtration, and recrystallised from water to yield the aldehyde (4.6g) in 76% yield.
References:
THE UNIVERSITY OF SUSSEX;MOORE, Anthony Lennox;ALBURY, Mary Susan;YOUNG, Luke Edward;ELLIOTT, Catherine WO2013/160670, 2013, A1 Location in patent:Page/Page column 24

504-15-4
404 suppliers
$7.00/100mg
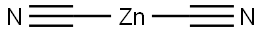
557-21-1
105 suppliers
$12.00/5g

487-69-4
78 suppliers
$24.00/250mg

504-15-4
404 suppliers
$7.00/100mg

487-69-4
78 suppliers
$24.00/250mg

504-15-4
404 suppliers
$7.00/100mg
50-00-0
892 suppliers
$10.00/25g

487-69-4
78 suppliers
$24.00/250mg