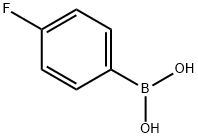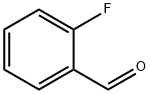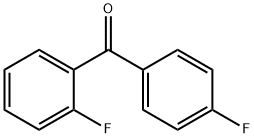
2,4'-Difluorobenzophenone synthesis
- Product Name:2,4'-Difluorobenzophenone
- CAS Number:342-25-6
- Molecular formula:C13H8F2O
- Molecular Weight:218.2

352-13-6
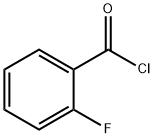
393-52-2

342-25-6
The general procedure for the synthesis of (2-fluorophenyl)(4-fluorophenyl)methanone from 4-fluorophenylmagnesium bromide and o-fluorobenzoyl chloride was as follows: 2-fluorobenzoyl chloride (6 mmol, 1.0 eq.) was dissolved in 2-methyltetrahydrofuran (15 mL) and transported through the material channel A and the metering pump P1; meanwhile, 4-fluorophenylmagnesium bromide (9 mmol, 1.5 eq.) was dissolved in 2- methyltetrahydrofuran (15 mL), delivered through material channel B and metering pump P2. The two solutions were mixed in the mixing module M at a preset temperature of 25 °C and a flow rate of 1 mL/min, and then entered into the reaction module L for 1 hour. Upon completion of the reaction, the reaction solution flowed out of outlet D and was collected in 1 mol/L hydrochloric acid solution for quenching. The quenched solution was stirred, washed with saturated brine solution, dried with anhydrous sodium sulfate, concentrated and purified by column chromatography to afford the target product (2-fluorophenyl)(4-fluorophenyl)methanone.
Yield:342-25-6 98%
Reaction Conditions:
with potassium phosphate;palladium diacetate in water;methyl cyclohexane; for 8 h;Reflux;Solvent;
Steps:
3 Example 3
Method of the present embodiment provides a 2,4 ′-difluorobenzophenone and preparation method thereof, as follows: in a 1000 ml round bottom flask The bottle was added sequentially o-fluoro benzaldehyde 124 g of 4-fluorophenylboronic acid and 140 g of potassium phosphate, 106 grams, 1.12 g of palladium acetate, then adding 500 ml of methyl cyclohexane and 100 ml of water and heated to reflux for 8 hours., sequentially passes through filtering, layering, washing with water, removing the solvent to obtain the product., 2,4 '-difluorobenzophenone 214 g, 98% yield.
References:
CN104230691,2016,B Location in patent:Paragraph 0028-0029

352-13-6
202 suppliers
$40.00/50mL

393-52-2
398 suppliers
$10.00/25g

342-25-6
306 suppliers
$6.00/5g
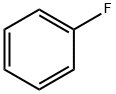
462-06-6
430 suppliers
$10.00/5g
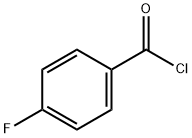
403-43-0
481 suppliers
$9.00/25g
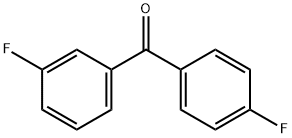
345-71-1
37 suppliers
$20.00/250mg

342-25-6
306 suppliers
$6.00/5g
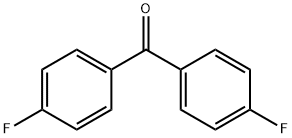
345-92-6
582 suppliers
$10.00/1g
56-23-5
0 suppliers
$25.00/10ml

462-06-6
430 suppliers
$10.00/5g

342-25-6
306 suppliers
$6.00/5g

345-92-6
582 suppliers
$10.00/1g