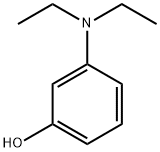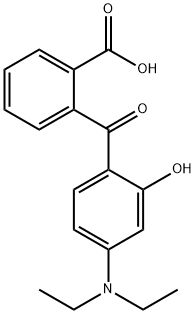
2-(4-Diethylamino-2-hydroxybenzoyl)benzoic acid synthesis
- Product Name:2-(4-Diethylamino-2-hydroxybenzoyl)benzoic acid
- CAS Number:5809-23-4
- Molecular formula:C18H19NO4
- Molecular Weight:313.35

59404-96-5

5809-23-4
To a stirred solution of compound 2 (1.0 eq.) in CH2Cl2 was slowly added a solution of BBr3 (1.9 eq.) in CH2Cl2 at -78 °C. After 1 hour of reaction, the mixture was slowly warmed to -25°C. Upon completion of the reaction, the reaction mixture was quenched with H2O and the solvent was subsequently evaporated to give the crude product. For compounds 3a to 3c, the pure product was obtained by recrystallization from MeOH/H2O solution as a yellow solid, while compound 3d was purified by silica gel column chromatography to give a yellow viscous oil. The 1H-NMR (CDCl3, 500 MHz) data for compound 3a (2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid) were as follows: δ 12.65 (s, 1H), 8.07 (dd, 1H, J = 7.8, 0.9Hz), 7.58 (td, 1H, J = 7.6, 1.4Hz), 7.51 (td, 1H, J = 7.8, 1.4Hz), 7.51 (td, 1H, 1H, J = 7.6, 1.4Hz). J = 7.8, 1.4Hz), 7.34 (dd, 1H, J = 7.6, 1.1Hz), 6.91 (d, 1H, J = 8.9Hz), 6.11 (sd, 1H, J = 2.5Hz), 6.03 (dd, 1H, J = 9.2, 2.5Hz), 3.37 (q, 4H, J = 7.1Hz), 1.18 (t, 6H, J = 7.1 Hz).13C-NMR (CDCl3, 500 MHz) data were as follows: δ 199.0, 167.5, 165.2, 153.6, 140.6, 134.6, 131.6, 130.4, 129.5, 128.9, 127.6, 110.1, 103.5, 96.7, 124.5, 12.5. HRMS (EI) calculated value C19H21NO4 (M+): 313.1314, measured value: 313.1313. yield was 65%.
Yield:5809-23-4 98%
Reaction Conditions:
Stage #1: phthalic anhydride;3-diethylaminophenol in toluene;Inert atmosphere;Reflux;
Stage #2: with sodium hydroxide in toluene at 50 - 90; for 6 h;Inert atmosphere;
Steps:
2-Carboxyl-4’-diethylamino-2’-hydroxy benzophenone (1).
3-Diethylamino phenol(10.00 g, 60.52 mmol) and phthalic anhydride (8.96 g, 60.52 mmol) in toluene (50 mL) wererefluxed under nitrogen for 4 h. The mixture was cooled to 50-60oC. 50mL of 35% NaOH (w/w)aqueous solution was added and then heated to 90oC for 6 h. The resulting mixture was pouredinto 200 g ice, acidified with concentrated HCl, and allowed to stand at room temperature for2 h. The suspension was filtered, and the solid was recrystallized from ethanol, then dried toafford the desired product (18.60 g) in a 98% yield.
References:
He, Haihong;He, Tingting;Zhang, Ziqian;Xu, Xiu;Yang, Huibin;Qian, Xuhong;Yang, Youjun [Chinese Chemical Letters,2018,vol. 29,# 10,p. 1497 - 1499] Location in patent:supporting information

59404-96-5
0 suppliers
inquiry

5809-23-4
198 suppliers
$5.00/1g
81-88-9
502 suppliers
$24.39/50g

5809-23-4
198 suppliers
$5.00/1g
85-44-9
778 suppliers
$9.00/5g

5809-23-4
198 suppliers
$5.00/1g

92-18-2
15 suppliers
inquiry

5809-23-4
198 suppliers
$5.00/1g