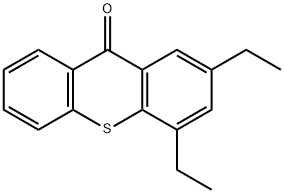
2,4-Diethyl-9H-thioxanthen-9-one synthesis
- Product Name:2,4-Diethyl-9H-thioxanthen-9-one
- CAS Number:82799-44-8
- Molecular formula:C17H16OS
- Molecular Weight:268.37

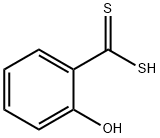
141-93-5
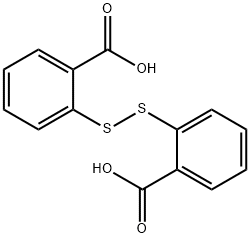
119-80-2
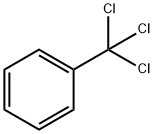
98-07-7

82799-44-8
Under nitrogen protection, 300 mL of anhydrous toluene, 28.6 g of trichloromethylbenzene, 86 mg of anhydrous ferric chloride and 20.4 g of dithiosalicylic acid were added to the reaction flask. The reaction system was heated to 100 °C and stirred continuously until thin-layer chromatography (TLC) detection showed that the raw materials had largely disappeared. Subsequently, residual chlorine or hydrogen chloride gas was removed from the system by nitrogen bubbling. The solvent was recovered by distillation under reduced pressure and the residue was distilled under reduced pressure to give 17.6 g of benzoyl chloride. The remaining o-chlorothiobenzoyl chloride intermediate in the flask was mixed with 20.3 g of diethylbenzene and 200 mL of dichloroethane and added slowly and dropwise to a 150 mL solution of dichloroethane containing 23.3 g of anhydrous aluminum trichloride powder. The reaction temperature was maintained at 20°C and the reaction was carried out under effective stirring for half an hour. Subsequently, the reaction mixture was slowly poured into an equal volume of ice-water mixture under rapid mechanical stirring. The organic phase was washed sequentially with water and dilute sodium hydroxide solution and the solvent was evaporated to dryness to give an oily substance. The oil was recrystallized from hot methanol to give 28.6 g of 2,4-diethylthiazolone (DETX).

141-93-5
134 suppliers
$44.00/2ml

119-80-2
438 suppliers
$7.00/25g

82799-44-8
285 suppliers
$5.00/5g
Yield:82799-44-8 90.2%
Reaction Conditions:
with sulfuric acid at 0 - 65; for 6 h;Reagent/catalyst;
Steps:
1-2 Example one
Add 40 ml of concentrated sulfuric acid to a 500 ml four-neck reaction flask.100 grams of dithiosalicylic acid,8 grams of polyphosphate,With stirring in an ice-salt bath to cool below 0 ° C, 100 g of 1,3-diethylbenzene was added dropwise to control the reaction temperature not to exceed 5 ° C.After the addition was completed, the ice-salt bath was removed, the temperature was naturally raised to room temperature, and the reaction was continued for 1 hour.The oil bath was replaced, the temperature was slowly raised to 65 ° C, and the reaction was performed at 65 ° C for 5 hours. The reaction was followed by HPLC until the dithiosalicylic acid reaction was completed.The reaction product was cooled, 90 g of 1,3-diethylbenzene and 60 g of water were added, and the mixture was stirred for half an hour, and the phases were separated. The organic phase was washed with alkali, washed with water, concentrated, and then cooled to 5 ° C.Overnight, the next day was filtered, the filter cake was washed with a small amount of ice ethanol, and dried in vacuum.150 g of bright yellow crystalline powder, namely DETX finished product,Yield 90.2%,Melting point 71 72 ,The purity of the product by HPLC was 99.6%.
References:
Ji'an Dongqing Fine Chemical Co., Ltd.;Ceng Xiaojun CN110845471, 2020, A Location in patent:Paragraph 0008; 0027-0030

141-93-5
134 suppliers
$44.00/2ml

119-80-2
438 suppliers
$7.00/25g

98-07-7
333 suppliers
$13.00/25g

82799-44-8
285 suppliers
$5.00/5g