
2,4-Dichloro-5-fluoropyrimidine synthesis
- Product Name:2,4-Dichloro-5-fluoropyrimidine
- CAS Number:2927-71-1
- Molecular formula:C4HCl2FN2
- Molecular Weight:166.97
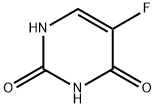
51-21-8

2927-71-1
The general procedure for the synthesis of 2,4-dichloro-5-fluoropyrimidine using 5-fluoropyrimidine-2,4(1H,3H)-dione as a starting material is as follows: 5-fluoropyrimidine-2,4-diol (525 kg, 1.00 mole equivalents) was mixed with phosphorus trichloride (1545 kg, 2.50 mole equivalents) under nitrogen protection and heated to about 100 °C with stirring. Subsequently, N,N-dimethylaniline (980 kg, 2.00 mole equivalents) was slowly added over a period of about 9 hours and the reaction mixture was continued to be stirred at about 100°C for no more than 4 hours. Upon completion of the reaction, the mixture was cooled to about 20°C over about 2 hours and then the reaction was quenched by slowly pouring into a mixture of water (3150 kg) and dichloromethane (1915 kg), ensuring that the temperature was controlled below 40°C. The mixture was stirred at about 20°C for at least 3 hours and then left to stratify. The aqueous phase was washed with dichloromethane (1915 kg) and the layers were separated again. The organic phase was combined and washed at least once with concentrated aqueous hydrochloric acid (525 kg), repeated if necessary, and then with 5% w/w aqueous sodium bicarbonate (2625 kg). The washed organic solution was concentrated by distillation at atmospheric pressure to about 1310 kg to give a dichloromethane solution of 2,4-dichloro-5-fluoropyrimidine with a typical concentration of about 50% w/w and a yield of about 95%. The solution can be used directly in subsequent reactions.

51-21-8
837 suppliers
$5.00/5g

2927-71-1
435 suppliers
$6.00/5g
Yield:2927-71-1 95%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-aniline at 100; for 13 h;Inert atmosphere;Large scale;
Steps:
1.A
5-fluoropyrimidine-2,4-diol (525 kg, 1.00 mol eq) is mixed with phosphorous oxychloride (1545 kg, 2.50 mol eq) and heated to about 100° C. with stirring under a nitrogen atmosphere. N,N-dimethylaniline (980 kg, 2.00 mol eq) is then added over a period of about 9 hours and the resulting mixture stirred at about 100° C. for up to 4 hours.
This is then cooled to about 20° C. over about 2 hours and then quenched into a mixture of water (3150 kg) and dichloromethane (1915 kg), maintaining the temperature below 40° C.
The contents are then stirred at about 20° C. for at least 3 hours and then the layers separated.
The aqueous phase is washed with dichloromethane (1915 kg) and the layers again separated.
The combined organics are then washed with concentrated aqueous hydrochloric acid (525 kg) at least once, sometimes more than once, then with 5% w/w aqueous sodium hydrogen carbonate solution (2625 kg).
The resulting organic solution is then distilled at atmospheric pressure down to about 1310 kg to give a solution of 2,4-dichloro-5-fluoro-pyrimidine in dichloromethane, with typical solution strength of about 50% w/w and yield of about 95%.
This solution is then used directly in the next process.
References:
McKeever, Benedict;Diorazio, Louis Joseph;Jones, Martin Francis;Ferris, Leigh;Janbon, Sophie Laure Marie;Siedlecki, Pawel Stanislaw;Churchill, Gwydion Huw;Crafts, Peter Alan US2015/175639, 2015, A1 Location in patent:Paragraph 0145

4330-22-7
37 suppliers
inquiry

2927-71-1
435 suppliers
$6.00/5g

51-21-8
837 suppliers
$5.00/5g

2927-71-1
435 suppliers
$6.00/5g
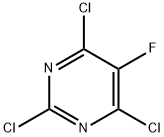
6693-08-9
105 suppliers
$26.00/100mg

2927-71-1
435 suppliers
$6.00/5g
74-96-4
458 suppliers
$9.00/5g

2927-71-1
435 suppliers
$6.00/5g