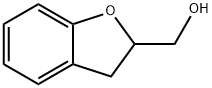
2,3-DIHYDRO-1-BENZOFURAN-2-YLMETHANOL synthesis
- Product Name:2,3-DIHYDRO-1-BENZOFURAN-2-YLMETHANOL
- CAS Number:66158-96-1
- Molecular formula:C9H10O2
- Molecular Weight:150.17
1745-81-9

66158-96-1
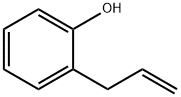
2-Allylphenol (5) (200 mg, 1.49 mmol) was used as a raw material, which was mixed with m-chloroperoxybenzoic acid (616 mg, 3.57 mmol) in dichloromethane (DCM, 30 mL) and the reaction was stirred for 6 hours at room temperature. Subsequently, potassium carbonate (K2CO3, 617 mg, 4.47 mmol) and methanol (25 mL) were added to the reaction system and stirring was continued for 14 hours. After completion of the reaction, the solvent was removed by evaporation and diluted by adding water (15 mL). The mixture was extracted with dichloromethane (3 x 20 mL), the organic phases were combined, washed with brine (10 mL), dried over anhydrous sodium sulfate (Na2SO4), and the solvent was evaporated again. The crude product was purified by fast column chromatography (eluent: ethyl acetate/hexane=1:7) to afford the target compound (2,3-dihydrobenzofuran-2-yl)methanol (6) (195 mg, 87% yield) as a light yellow oil. The structure of the product was confirmed by 1H NMR (CDCl3, 300 MHz) and FTIR (neat): 1H NMR δ 7.17 (d, 1H, J = 7.4 Hz), 7.11 (t, 1H, J = 7.6 Hz), 6.85 (t, 1H, J = 7.4 Hz), 6.79 (d, 1H, J = 7.4 Hz), 4.91 (m, 1H) , 3.85 (dd, 1H, J = 12.0, 3.2 Hz), 3.74 (dd, 1H, J = 12.0, 6.3 Hz), 3.25 (dd, 1H, J = 15.5, 9.4 Hz), 3.01 (dd, 1H, J = 15.5, 7.4 Hz); FTIR 3391, 3049, 1047 cm-1.

1745-81-9
289 suppliers
$14.00/5g

66158-96-1
63 suppliers
inquiry
Yield:66158-96-1 87%
Reaction Conditions:
Stage #1: o-Allylphenolwith 3-chloro-benzenecarboperoxoic acid in dichloromethane; for 6 h;Inert atmosphere;
Stage #2: with methanol;potassium carbonate in dichloromethane at 20; for 14 h;Inert atmosphere;
Steps:
Genera lProcedure for the synthesis of (2,3-Dihydrobenzofuran-2-yl)-methanol (6)
A mixture of 2-allylphenol (5) (200 mg, 1.49 mmol), m-chloroperoxybenzoic acid (616 mg, 3.57 mmol) and dichloromethane (DCM, 30 mL) was stirred for 6 h. then K2CO3 (617 mg, 4.47 mmol) and methanol (25 mL) was added at room temperature. After stirring for 14 h, solvent was evaporated then water (15 mL) was added. The mixture was extracted with DCM (3× 20 mL). The combined extracts were washed with brine (10 mL), dried over anhydrous Na2SO4 and the solvent was evaporated.Purification via flash column chromatography (EtOAc/hexane = 1:7) provided (2,3-dihydrobenzofuran-2-yl)-methanol (6) (195 mg, 87%) as a pale yellow oil. 1H NMR (CDCl3, 300 MHz) δ 7.17 (d, 1H, J = 7.4Hz), 7.11 (t, 1H, J = 7.6 Hz), 6.85(t, 1H, J = 7.4 Hz), 6.79 (d, 1H, J = 7.4 Hz), 4.91 (m, 1H), 3.85 (dd, 1H,J = 12.0, 3.2 Hz), 3.74 (dd, 1H, J = 12.0, 6.3 Hz), 3.25 (dd, 1H, J = 15.5, 9.4 Hz), 3.01 (dd, 1H, J = 15.5, 7.4 Hz); FTIR (neat) 3391, 3049, 1047 cm-1
References:
Choi, Minho;Jo, Hyeju;Park, Hyun-Jung;Sateesh Kumar, Arepalli;Lee, Joonkwang;Yun, Jieun;Kim, Youngsoo;Han, Sang-Bae;Jung, Jae-Kyung;Cho, Jungsook;Lee, Kiho;Kwak, Jae-Hwan;Lee, Heesoon [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 12,art. no. 22633,p. 2545 - 2549] Location in patent:supporting information
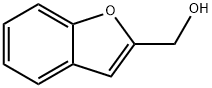
55038-01-2
94 suppliers
$132.00/1g

66158-96-1
63 suppliers
inquiry
1746-13-0
242 suppliers
$9.00/5g

66158-96-1
63 suppliers
inquiry
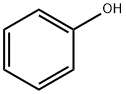
108-95-2
768 suppliers
$14.00/25g

66158-96-1
63 suppliers
inquiry
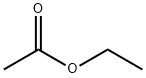
141-78-6
665 suppliers
$22.37/250ml

1745-81-9
289 suppliers
$14.00/5g

66158-96-1
63 suppliers
inquiry