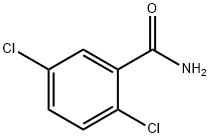
2,3-DICHLOROBENZAMIDE synthesis
- Product Name:2,3-DICHLOROBENZAMIDE
- CAS Number:5980-26-7
- Molecular formula:C7H5Cl2NO
- Molecular Weight:190.03
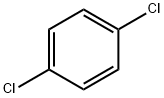
106-46-7
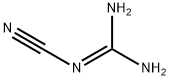
127099-85-8

5980-26-7
GENERAL SYNTHESIS: Cyanoguanidine (0.084 g, 1 mmol) was suspended in 2 mL of pure aromatic solvent (or the aromatic hydrocarbon can be suspended in CH2Cl2) and freshly distilled trifluoromethanesulfonic acid (0.5 mL, 6 mmol) was added slowly and dropwise. The reaction mixture was stirred at 60 °C for 2 h, followed by the addition of 1 mL of cold water. Stirring of the mixture was continued overnight. Product separation was accomplished by alkalinizing the reaction mixture with 10 M NaOH and extracting twice with chloroform. The organic phases were combined, washed sequentially with water and saturated saline and dried over anhydrous MgSO4. The crude product can be further purified by silica gel column chromatography (eluent: hexane/ethyl acetate).
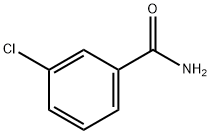
618-48-4
113 suppliers
$12.00/1g

5980-26-7
99 suppliers
$5.00/250mg
Yield: 78%
Reaction Conditions:
with N-chloro-succinimide;palladium diacetate;trifluoroacetic acid in 1,2-dichloro-ethane at 90; for 24 h;Sealed tube;regioselective reaction;
Steps:
2.1. General procedure for palladium(II)-catalyzed ortho-halogenation of benzamides
General procedure: To a clean oven-dried 15 mL sealed tube equipped with magnetic stir bar, benzamide (0.25 mmol, 1.0 equiv), Pd(OAc)2 (5.0 mol%, 2.8 mg), and NXS (0.3 mmol, 1.2 equiv.) were added sequentially. DCE (2.0 mL) was then added to the reaction mixture followed by trifluoroacetic acid (475 μL). The tube was tightly closed and placed in a preheated oil bath of 60 °C and stirred for 24 h. In each case, the reaction was monitored by TLC, and after completion, the reaction mixture was cooled to room temperature. The solvent was evaporated under reduced pressure and then diluted with ethyl acetate followed by neutralization with a saturated solution of sodium bicarbonate. After extraction with ethyl acetate (15 mL×3), the organic layer was washed with brine solution and dried over sodium sulphate. After evaporation of the solvent, the crude mixture was purified by column chromatography silica gel using ethyl acetate/hexanes as the eluent.
References:
Jaiswal, Yogesh;Kumar, Amit [Catalysis Communications,2019,vol. 131,art. no. 105784]
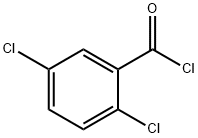
2905-61-5
187 suppliers
$15.00/250mg

5980-26-7
99 suppliers
$5.00/250mg
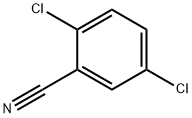
21663-61-6
235 suppliers
$12.00/5g

5980-26-7
99 suppliers
$5.00/250mg
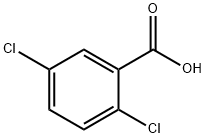
50-79-3
332 suppliers
$7.00/5g

5980-26-7
99 suppliers
$5.00/250mg