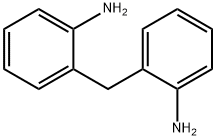
2,2'-methylenedianiline synthesis
- Product Name:2,2'-methylenedianiline
- CAS Number:6582-52-1
- Molecular formula:C13H14N2
- Molecular Weight:198.26
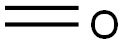
50-00-0

142-04-1
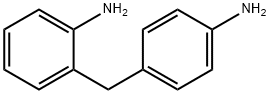
1208-52-2

6582-52-1
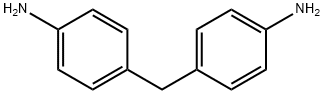
101-77-9
The general procedure for the synthesis of 2-(4-aminobenzyl)aniline, 2,2'-methylenedianiline and 4,4'-diaminodiphenylmethane from formaldehyde and aniline hydrochloride is as follows: 1. hydrochloric acid from tank 2 (at a concentration of 30.8 wt%, which is a by-product of the MDI unit) and aniline from tank 3 are transported via pump 6 in a ratio of hydrochloric acid to aniline at a molar ratio of 0.36:1 to a venturi mixer 5, wherein mixing is carried out and reaction is carried out to generate aniline hydrochloride. Subsequently, the generated aniline hydrochloride is pumped into a recirculation tube and mixed with the recirculating solution from the condensing mixing vessel 1 to form a mixed solution. 2. The mixed solution is introduced into a heat exchanger 7, where the heat of reaction is removed, cooled to 38°C, and then conveyed to the inlet of a high gravity rotary bed reactor 8. 3. at the same time, the formaldehyde solution (with a concentration of 37 wt%) from the storage tank 4 was fed through the other feed port of the high gravity rotary bed reactor 8, controlling the molar ratio of formaldehyde to aniline to be 0.40:1. in the high gravity rotary bed reactor 8, the formaldehyde solution was fully mixed with the premixed solution and underwent a pre-condensation reaction under the following reaction conditions: temperature 35°C, reaction time 0.5 sec, rotor speed 1000 rpm. 4. The mixed solution after reaction flows into the condensation reactor 1 for further pre-condensation reaction under the following conditions: temperature 42°C, stirring speed 110 rpm, reaction residence time 20 minutes. 5. Subsequently, the temperature of the reaction solution was raised to above 90 °C for a molecular rearrangement reaction with a reaction residence time of about 2 h. A solution of diphenylmethylene diamine hydrochloride and polymethylene polyphenylene polyamine hydrochloride was finally obtained. 6. Of the reaction mixture from the condensing reactor 1, 92 vol% was returned to the recirculation tubes as a recirculating solution and flowed through the heat exchanger 7, while the remaining 8 vol% was discharged and neutralized with a sodium hydroxide solution at a concentration of 42 wt%. 7. The brine phase was separated from the polyamine organic phase, the polyamine organic phase was washed with water and purified to give a final mixture of diphenylmethylene diamine and polymethylene polyphenylene polyamine. The product composition is detailed in Table 2.
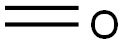
50-00-0
892 suppliers
$10.00/25g

142-04-1
208 suppliers
$10.00/10g

1208-52-2
92 suppliers
$75.00/25 mg

6582-52-1
42 suppliers
inquiry

101-77-9
365 suppliers
$15.00/25g
Yield: 66.75 %Chromat. , 0.085 %Chromat. , 4.30 %Chromat.
Reaction Conditions:
Stage #1:formaldehyd;aniline hydrochloride in water at 38 - 90;
Stage #2: with sodium hydroxide in waterProduct distribution / selectivity;
Steps:
1
Example 1:; Hydrochloric acid (a concentration percentage by weight is 30.8%, this hydrochloric acid is a by-product from MDI apparatus) from storage tank 2 and aniline from storage tank 3 are fed into venturi mixer 5 by a pump 6 with a molar ratio of hydrochloric acid/anilin=0.36:1, for mixing and reacting with each other to produce aniline hydrochloride which is then pumped into circulation pipes and mixed with a circulation solution coming from a condensation stirred vessel 1 to obtain a mixed solution. The obtained mixed solution is introduced into a heat exchanger 7 to remove the reaction heat, and the mixed solution, which is cooled to 38°C and left the heat exchanger 7, is introduced into a feeding port of the high gravity rotating bed reactor 8 of rotating packed bed type. Formaldehyde solution (a concentration percentage by weight is 37 wt%) stream from storage tank 4 is fed through another feeding port of the high gravity rotating bed reactor 8, the ratio of formaldehyde to aniline is controlled at 0.40:1. The formaldehyde solution is mixed sufficiently with the previously mixed solution phase and conducted a pre-condensation reaction in the high gravity rotating bed reactor 8, the reaction temperature is controlled at 35°C, the reaction time is 0.5 sec, and the rotation speed of the rotor of high gravity rotating bed reactor is 1000rpm. Then the mixed reaction solution flows into the condensation reaction vessel 1 to proceed with the pre-condensation reaction, the temperature of reaction solution is controlled at 42°C, the stirring speed is about 110rpm, and the reaction residence time is about 20 min. Then the temperature of reaction solution is elevated to over 90°C to conduct a molecular rearrangement reaction, the residence time for molecular rearrangement reaction is about 2 hours. Finally a solution of diphenylmethylene diamine hydrochloride and polymethylene polyphenyl polyamines hydrochloride is obtained. 92 vol% of the reaction mixture from the condensation reaction vessel 1 returns to the circulation pipes, as circulation solution, and flows to heat exchanger 7, the other 8 vol% of the reaction mixture is discharged and neutralized with a sodium hydroxide solution at a concentration of 42 wt%. The salt water phase is separated from the polyamine organic phase, and the polyamines are washed with water and purified to finally obtain a mixture of diphenylmethylene diamine and polymethylene polyphenyl polyamines. The composition of products is listed in table 2.
References:
Ningbo Wanhua Polyurethanes Co., Ltd.;Beijing University Of Chemical Technology EP2145874, 2010, A1 Location in patent:Page/Page column 6
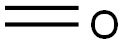
50-00-0
892 suppliers
$10.00/25g
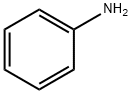
62-53-3
689 suppliers
$10.00/1g

6582-52-1
42 suppliers
inquiry

101-77-9
365 suppliers
$15.00/25g
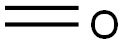
50-00-0
892 suppliers
$10.00/25g

62-53-3
689 suppliers
$10.00/1g

1208-52-2
92 suppliers
$75.00/25 mg

6582-52-1
42 suppliers
inquiry

101-77-9
365 suppliers
$15.00/25g
![1-nitro-2-[(2-nitrophenyl)methyl]benzene](/CAS/20180529/GIF/21540-57-8.gif)
21540-57-8
1 suppliers
inquiry

6582-52-1
42 suppliers
inquiry
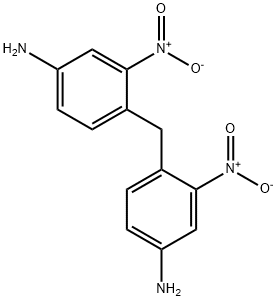
26946-33-8
14 suppliers
inquiry

6582-52-1
42 suppliers
inquiry