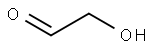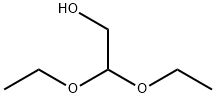
2,2-Diethoxyethanol synthesis
- Product Name:2,2-Diethoxyethanol
- CAS Number:621-63-6
- Molecular formula:C6H14O3
- Molecular Weight:134.17
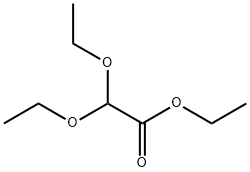
6065-82-3

621-63-6
The general procedure for the synthesis of 2,2-diethoxyethanol from ethyl 2,2-diethoxyacetate was as follows: 302.6 g (8 mol) of sodium borohydride was slowly added to 2 L of 1,2-dimethoxyethane (monoglycol dimethyl ether) under stirring conditions. Subsequently, 704.8 g (4 mol) of ethyl 2,2-diethoxyacetate solution dissolved in 4 L of ethanol was added dropwise, and the dropwise acceleration was controlled to maintain the reaction temperature below 50 °C, a process that took about 4 hours. After completion of the titration, the reaction mixture was heated to reflux for 3 hours. Upon completion of the reaction, about 2 L of ethanol was removed by distillation, followed by the slow dropwise addition of 4 L of water, while residual ethanol was removed by azeotropic distillation. During the dropwise addition, a large amount of precipitate was observed to be generated, but the precipitate gradually dissolved with continued addition of water. Next, 1,2-dimethoxyethane was added to the reaction mixture and the mixture was cooled in an ice bath. Under stirring, 600 g of potassium carbonate was added to the cooled mixture. Subsequently, the mixture was extracted with 2 L of ether and the organic phases were combined and dried with anhydrous magnesium sulfate. After drying, the ether was removed by evaporation to give the crude product. The crude product was purified by vacuum distillation and the fractions with boiling points of 75-76 °C (15 mmHg) were collected to give 475.8 g of the target product 2,2-diethoxyethanol in 88.7% yield. The product was analyzed by elemental analysis (calculated values: C 53.7%, H 10.5%; measured values: C 53.11%, H 10.57%) and IR spectroscopy (3441 cm^-1; 2976 cm^-1; 2931 cm^-1; 2883 cm^-1; 1445 cm^-1; 1374 cm^-1; 1345 cm^-1; 1235 cm^- 1; 1134 cm^-1; 1073 cm^-1, KBr compression method) characterization confirmed.

6065-82-3
225 suppliers
$5.00/1g

621-63-6
198 suppliers
$10.00/1g
Yield:621-63-6 88.7%
Reaction Conditions:
with sodium tetrahydroborate in dimethoxyethane, 1,2;ethanol at 50; for 7 h;Heating / reflux;
Steps:
F Example F. 2,2-Diethoxy-ethanol.
302,6 g (8 mol) of sodium borohydride was added to 2 1 of 1,2-dimethoxyethane (monoglyme) with stirring, after which 704,8 g (4 mol) ethyl diethoxyacetate dissolved in 4 1 of ethanol was added dropwise within 4 hours so that the temperature was kept below 50 °C. The mixture was then heated to reflux for 3 hours. Then 2 1 of ethanol was distilled off, and 4 1 of water was added dropwise while the remaining ethanol and then the 1,2-dimethoxyethane was removed by distillation. During the water addition an abundant precipitate was formed which dissolved towards the end of the addition. The mixture was cooled on an icebath and 600 g of potassium carbonate was dissolved therein while stirring. The mixture was extracted with 2 1 of diethyl ether and dried with MgSO4. The diethyl ether was evaporated off and the crude product was distilled in vacuo at 75-76 °C (15 mm Hg). Yield = 475.8 g = 88.7 % [] Elemental analysis: CalculatedC 53.7%H 10.5%FoundC 53.11%H 10.57%IR: 3441 cm-1; 2976 cm-1; 2931 cm-1 ; 2883 cm-1; 1445 cm-1 1374 cm-1; 1345 cm-1; 1235 cm-1; 1134 cm-1; 1073 cm-1 (Between KBr plates)
References:
A/S GEA Farmaceutisk Fabrik EP1030841, 2004, B1 Location in patent:Page 11

60468-85-1
0 suppliers
inquiry

621-63-6
198 suppliers
$10.00/1g
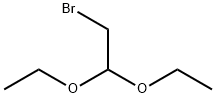
2032-35-1
489 suppliers
$5.00/10g

621-63-6
198 suppliers
$10.00/1g
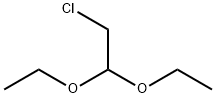
621-62-5
225 suppliers
$6.00/25g

621-63-6
198 suppliers
$10.00/1g