
1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)-
- CAS Number:143141-23-5
- Molecular formula:C13H10N2O2S
- Molecular Weight:258.3
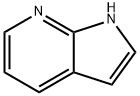
271-63-6
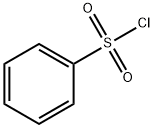
98-09-9
![1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)-](/CAS/GIF/143141-23-5.gif)
143141-23-5
4.2.4 Synthesis of 1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine (5) In a dry and nitrogen purged round bottom flask, 1H-pyrrolo[2,3-b]pyridine (1.01 g, 8.58 mmol), tetrabutylammonium bromide (81 mg, 0.25 mmol), finely ground sodium hydroxide (1.02 g, 25.41 mmol) and dichloromethane (20 mL) were added. The mixture was cooled to 0 °C in an ice bath and stirred, followed by the slow dropwise addition of benzenesulfonyl chloride (1.35 mL, 10.59 mmol). The reaction mixture was gradually warmed to room temperature and stirred continuously at this temperature for 1 hour. Upon completion of the reaction, water (20 mL) was added for hydrolysis and then extracted twice with dichloromethane. The organic layers were combined, washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give 2.38 g of a beige solid. The crude product was purified by silica gel column chromatography (eluent: cyclohexane/ethyl acetate=7:3) to give 2.17 g of white solid target product in 99% yield. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.45 (dd, J = 1.5, 4.8 Hz, 1H), 8.20 (d, J = 7.5 Hz, 2H), 7.86 (dd, J = 1.5, 7.8 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.57 (dd, J = 7.4 Hz, 1H ), 7.52-7.47 (m, 2H), 7.19 (dd, J = 4.8, 7.8 Hz, 1H), 6.61 (d, J = 4.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm): 144.7 (2C), 138.3, 134.1, 129.9, 129.1 (2C), 128.0 (2C), 126.5, 123.0, 119.0, 105.5.

271-63-6
583 suppliers
$9.00/5g

98-09-9
363 suppliers
$12.00/25 g
![1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)-](/CAS/GIF/143141-23-5.gif)
143141-23-5
83 suppliers
$30.00/1 g
Yield: 99%
Reaction Conditions:
Stage #1:7-Azaindole with tetrabutylammomium bromide;sodium hydroxide in dichloromethane
Stage #2:benzenesulfonyl chloride in dichloromethane at 0 - 20;
Steps:
4 4.2.4. 1-(Phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine (5)
4.2.4
1-(Phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine (5)
In a heat dried and nitrogen purged round bottom flask, 1H-pyrrolo[2,3-b]pyridine 1 (1.01 g, 8.58 mmol), tetrabutylammonium bromide (81 mg, 0.25 mmol), finely grounded sodium hydroxide (1.02 g, 25.41 mmol) and CH2Cl2 (20 mL) were mixed, stirred and cooled down to 0 °C in an ice bath, then benzene sulfonylchloride (1.35 mL, 10.59 mmol) was added slowly.
The mixture was left to warm up to room temperature and stirred at this temperature for 1 h.
The reaction was hydrolysed with water (20 mL) and extracted by CH2Cl2 (twice).
The organic layer was washed with a saturated sodium chloride solution, dried over magnesium sulfate and concentrated under reduced pressure to give 2.38 g of a beige solid.
The crude product was purified by chromatography on silica gel column, cyclohexane/EtOAc 7:3, to give 2.17 g of the pure expected product as a white solid in 99% yield. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.45 (dd, J = 1.5, 4.8 Hz, 1H), 8.20 (d, J = 7.5 Hz, 2H), 7.86 (dd, J = 1.5, 7.8 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.57 (dd, J = 7.4 Hz, 1H), 7.52-7.47 (m, 2H), 7.19 (dd, J = 4.8, 7.8 Hz, 1H), 6.61 (d, J = 4.0 Hz, 1H).
13C NMR (75 MHz, CDCl3) δ (ppm): 144.7 (2C), 138.3, 134.1, 129.9, 129.1 (2C), 128.0 (2C), 126.5, 123.0, 119.0, 105.5.
References:
Baltus, Christine B.;Jorda, Radek;Marot, Christophe;Berka, Karel;Bazgier, Václav;Kry?tof, Vladimír;Prié, Gildas;Viaud-Massuard, Marie-Claude [European Journal of Medicinal Chemistry,2016,vol. 108,p. 701 - 719]

271-63-6
583 suppliers
$9.00/5g
![1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)-](/CAS/GIF/143141-23-5.gif)
143141-23-5
83 suppliers
$30.00/1 g