ethyl 1-benzyl-3-oxopiperidine-4-carboxylate hydrochloride synthesis
- Product Name:ethyl 1-benzyl-3-oxopiperidine-4-carboxylate hydrochloride
- CAS Number:175406-94-7
- Molecular formula:C14H17NO3
- Molecular Weight:247.29
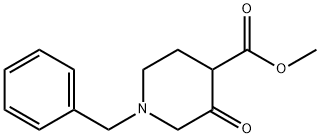
40114-49-6
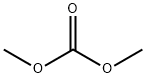
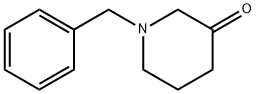
616-38-6

175406-94-7
To a stirred mixture of 1-benzyl-3-piperidone (72 g) and dimethyl carbonate (500 mL) was added sodium hydride (38 g, 60% oil dispersion) in batches. The reaction mixture was heated to reflux for 20 min and then the reaction was quenched by the slow addition of water (800 mL). The aqueous phase was extracted with ethyl acetate (3 x 400 mL), the organic phases were combined and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to give methyl 1-benzyl-3-oxopiperidine-4-carboxylate as a brown oil (93 g, 99% yield). lc-MS (ESI+): m/z 248 [M+H]+.

40114-49-6
162 suppliers
$90.00/10g

616-38-6
719 suppliers
$5.00/5 g

175406-94-7
42 suppliers
$79.00/250mg
Yield: 99%
Reaction Conditions:
with sodium hydride for 0.333333 h;Heating / reflux;
Steps:
51.1
[0248] To a stirred mixture of l-benzylpiperidin-3-one (72 g) and dimethyl carbonate (500 mL) was added NaH (38 g, 60%). After 20 minutes at reflux, water (800 mL) was added. The resulting solution was extracted with ethyl acetate (3 x 400 mL), dried over sodium sulfate and concentrated to give methyl l-benzyl-3- oxopiperidine-4-carboxylate as a brown oil (93 g, 99%). LCMS: 248 (M+H) +.
References:
KALYPSYS, INC. WO2009/26241, 2009, A1 Location in patent:Page/Page column 77-78

67-56-1
781 suppliers
$7.29/5ml-f
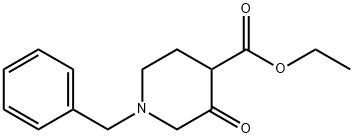
39514-19-7
135 suppliers
$17.00/1g

175406-94-7
42 suppliers
$79.00/250mg