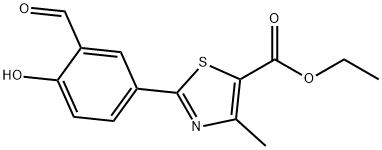
Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate synthesis
- Product Name:Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
- CAS Number:161798-01-2
- Molecular formula:C14H13NO4S
- Molecular Weight:291.32
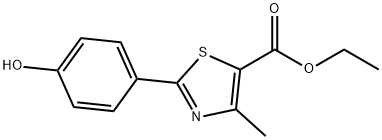
161797-99-5
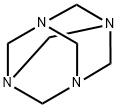
100-97-0

161798-01-2
The general procedure for the synthesis of ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate from ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate and urotropine was as follows: 120 kg of polyphosphoric acid was added to a clean and dry 200 L reactor, stirred and heated to 40-50°C. After stirring, 20 kg ( 76.0 mol) ethyl 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate and 11 kg (76.3 mol) hexamethylenetetramine (HMTA) were added sequentially. After the addition was completed, the reaction mixture was heated to 93°C for 3 hours. After completion of the reaction, the reaction solution was slowly poured into dilute aqueous glacial acetic acid solution for hydrolysis for 20 min. The reaction mixture was extracted with 300 L of ethyl acetate in three portions and the organic phases were combined. To the organic phase, 10 kg of anhydrous sodium sulfate was added for drying, and after filtration, the filtrate was concentrated under reduced pressure to obtain 210 mg of ethyl acetate solvent. To the concentrate, 100 kg of water was added and stirred to induce crystallization, followed by centrifugal separation and drying, resulting in 18.0 kg of light yellow intermediate product in 81.3% yield. The intermediate 2-(3-formyl-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylic acid ethyl ester was tested to be 99.4%, and the dicarboxylic acid impurity content was 0.008%.

161797-99-5
366 suppliers
$10.00/1g

100-97-0
0 suppliers
$14.00/250G

161798-01-2
381 suppliers
$6.00/250mg
Yield:161798-01-2 91.2%
Reaction Conditions:
with methanesulfonic acid;boric acid in cyclohexane at 75 - 100; for 7 h;Duff Aldehyde Synthesis;Temperature;Reagent/catalyst;Solvent;
Steps:
1-19 Example 13: Preparation of ethyl 2- (3-carbaldehyde-4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylate
(1) Add 1 mole portion of the reactant 2- (4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylic acid ethyl ester (actual dosage 80g) to (in a 1L reaction bottle) preheated to 100 In a homogeneous mixture of 1.5 molar parts of polyphosphoric acid and 2 molar parts of methanesulfonic acid, and 0.05 molar parts of cyclohexane and 0.002 molar parts of boric acid, stir well;
(2) To the above reaction mixture, 1.2 moles of Duff reaction reagent hexamethylenetetramine is added with stirring,Continue the reaction at a temperature of 75 ° C for 7 hours,After the reaction is completed, the reaction mixture is cooled to 38 ° C;
(3) Slowly add saturated ice brine (to about 80% of the total volume of the reaction flask) to the cooled reaction mixture,After the solid is precipitated, it is filtered, and the solid is washed with water until neutral and dried.The pale yellow solid is ethyl 2- (3-carbaldehyde-4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylate,The yield was 91.2%.
References:
CN111039891,2020,A Location in patent:Paragraph 0109-0184

161797-99-5
366 suppliers
$10.00/1g
64-19-7
1602 suppliers
$10.00/25ML

161798-01-2
381 suppliers
$6.00/250mg

25984-63-8
502 suppliers
$8.00/10g

161798-01-2
381 suppliers
$6.00/250mg
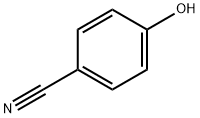
767-00-0
578 suppliers
$6.00/25g

161798-01-2
381 suppliers
$6.00/250mg
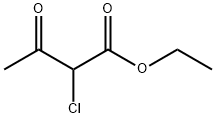
609-15-4
459 suppliers
$6.00/25g

161798-01-2
381 suppliers
$6.00/250mg