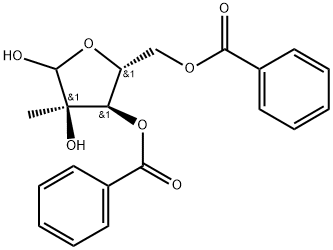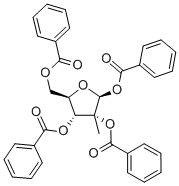
1,2,3,5-Tetra-O-benzoyl-2-C-methyl-beta-D-ribofuranose synthesis
- Product Name:1,2,3,5-Tetra-O-benzoyl-2-C-methyl-beta-D-ribofuranose
- CAS Number:15397-15-6
- Molecular formula:C34H28O9
- Molecular Weight:580.58

729596-46-7
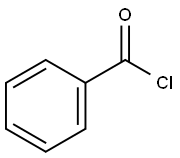
98-88-4

15397-15-6
1. 100 g (0.27 mol) of 3,5-di-O-benzoyl-2-C-methyl-D-ribofuranose was added to 300 mL of acetonitrile and stirred until completely dissolved. Subsequently 280 mL (2 mol) triethylamine was added. 2. 78.3 mL (0.67 mol) benzoyl chloride was added slowly dropwise at room temperature with controlled titration rate to avoid intense exotherm. 3. After the dropwise addition was completed, the reaction mixture was heated to 60 °C and maintained for 2 hours, during which time the progress of the reaction was monitored by HPLC. 4. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched by the addition of 150 mL of water. 5. The solid product was collected by filtration, washed with a mixture of acetonitrile/water (2:1, v/v), and subsequently dried under vacuum to constant weight to afford 109 g of 1,2,3,5-tetra-O-benzoyl-2-C-methyl-β-D-ribofuranose in 70% yield. 6. 8.25 kg (22.2 mol) 3,5-di-O-benzoyl-2-C-methyl-D-ribofuranose was dissolved in 21 kg of acetonitrile in a 100 L reactor. 7. 16.8 kg (166.5 mol) of triethylamine was added, followed by the slow dropwise addition of 9.8 kg (69.7 mol) of benzoyl chloride, taking care to control the reaction temperature. 8. The reaction mixture was heated to 60 °C and held for 4 h. 9. 9. After completion of the reaction, the mixture was cooled to room temperature and 13.7 L of water was added slowly and dropwise. 10. The mixture was further cooled to 0 °C and stirred continuously for 1 hour. 11. The solid product was separated by filtration and washed sequentially with 8 L of acetonitrile/water (2:1, v/v) mixture cooled to 0 °C and 4.7 kg of cold methanol.
Yield:15397-15-6 77%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
References:
Zhou, Longhu;Zhang, Hongwang;Tao, Sijia;Ehteshami, Maryam;Cho, Jong Hyun;McBrayer, Tamara R.;Tharnish, Philip;Whitaker, Tony;Amblard, Franck;Coats, Steven J.;Schinazi, Raymond F. [ACS Medicinal Chemistry Letters,2016,vol. 7,# 1,p. 17 - 22] Location in patent:supporting information

98-88-4
591 suppliers
$10.00/5g

15397-15-6
144 suppliers
$18.00/1g