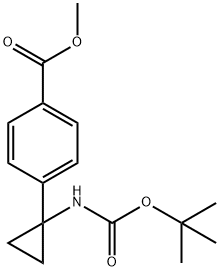
methyl 4-(1-((tert-butoxycarbonyl)amino)cyclopropyl)benzoate synthesis
- Product Name:methyl 4-(1-((tert-butoxycarbonyl)amino)cyclopropyl)benzoate
- CAS Number:1338243-88-1
- Molecular formula:C16H21NO4
- Molecular Weight:291.34

67-56-1
781 suppliers
$9.00/25ml

360773-84-8
76 suppliers
$29.00/250mg
201230-82-2
1 suppliers
inquiry

1338243-88-1
13 suppliers
inquiry
Yield:1338243-88-1 97%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 at 130;
Steps:
2 Step 2:
l-(N-boc-amino)-l-(4-bromophenyl)cyclopropane (10.5 g, 33.63 mmol) was carbonylated in MeOH (100 mL) at 130°C and 50 atm CO pressure with Pd(dppf)Cl2.DCM complex as catalyst. After consumption of the starting material, the resulting mixture was concentrated and the residue was partitioned between water (100 mL) and EtOAc (200 mL). The organic layer was collected, dried over sodium sulfate and concentrated to give methyl 4- (l-[(tert-butoxy)carbonyl]aminocyclopropyl)benzoate (9.5 g, 32.61 mmol, 97% yield) which was used in the next step without further purification.
References:
WO2020/221826,2020,A1 Location in patent:Page/Page column 165-166
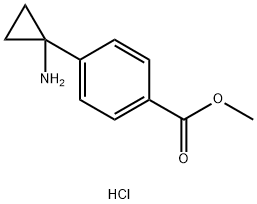
1014645-87-4
60 suppliers
$72.00/50mg

24424-99-5
864 suppliers
$13.50/25G

1338243-88-1
13 suppliers
inquiry
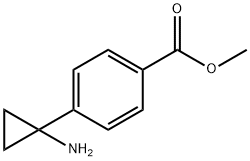
1006037-03-1
75 suppliers
$65.00/50mg

24424-99-5
864 suppliers
$13.50/25G

1338243-88-1
13 suppliers
inquiry
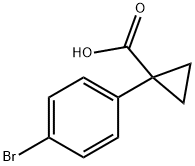
345965-52-8
170 suppliers
$7.00/250mg

1338243-88-1
13 suppliers
inquiry

24424-99-5
864 suppliers
$13.50/25G

1338243-88-1
13 suppliers
inquiry