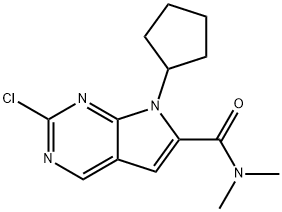
2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide synthesis
- Product Name: 2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide
- CAS Number:1211443-61-6
- Molecular formula:C14H17ClN4O
- Molecular Weight:292.76
![2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-carboxylic acid](/CAS/20150408/GIF/1211443-58-1.gif)
1211443-58-1
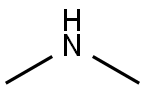
124-40-3
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
To a three-necked flask was added 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-D]pyrimidine-6-carboxylic acid (26.57 g, 100 mmol) and N,N-dimethylformamide (133 mL), and the mixture was cooled to 0-5 °C under stirring. EDCI (23.00 g, 120 mmol) and a tetrahydrofuran solution of dimethylamine (2.0 M, 75 mL, 150 mmol) were then added. Triethylamine (20.24 g, 200 mmol) was added dropwise and the mixture was stirred at 20-25 °C for 6-8 hours. After completion of the reaction, the reaction mixture was extracted three times with ethyl acetate (133 mL) and the organic phases were combined. The organic phase was sequentially washed twice with water and once with brine (133 mL), dried with anhydrous sodium sulfate and concentrated. Finally, the target product 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide (26.93 g, 92% yield) was purified by column chromatography in a mixed solvent of dichloromethane and methanol.
![2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-carboxylic acid](/CAS/20150408/GIF/1211443-58-1.gif)
1211443-58-1
133 suppliers
$23.00/100mg
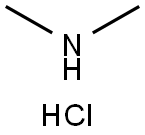
506-59-2
568 suppliers
$5.00/5G
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
197 suppliers
$18.00/250mg
Yield:1211443-61-6 99%
Reaction Conditions:
Stage #1: 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acidwith O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;triethylamine in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: N,N-dimethylammonium chloride in N,N-dimethyl-formamide at 20;Reagent/catalyst;
Steps:
1.1 Step 1 : (6-Chloro-l-cyclopentyl-l,5,7-triaza-lH-inden-2-yl)(dimethylamino)formaldehyde:
A stirring solution of 6-chloro-l-cyclopentyl-l,5,7-triaza-lH-indene-2-carboxylic acid (199 mg, 0.75 mmol) and triethylamine (631 L, 4.5 mmol) in DMF (4 mL) was treated with HBTU (427 mg, 1.13 mmol) in one portion. The resulting mixture was stirred at room temperature for 30 minutes. Dimethylamine hydrochloride salt (122 mg, 1.50 mmol) was added in one portion and the resulting solution was stirred at room temperature overnight. Next morning, LCMS analysis indicated clean conversion to product (m/z = 293.4). The reaction mixture was diluted with EtOAc, transferred to a separatory funnel and washed with 0.1 N HC1 aqueous and brine. The organics were dried over anhydrous MgS04, filtered and concentrated to yield the crude product. This was purified on a S1O2 column using a hexanes/EtOAc gradient eluent. The fractions containing the product were concentrated to yield 215 mg (0.74 mmol, 99 %). LC/MS - HPLC (254 nm) - Rt 2.90 min. MS (ESI) m/z 293.4 [M+ + H+].
References:
WO2018/140730,2018,A1 Location in patent:Paragraph 0218
![2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-carboxylic acid](/CAS/20150408/GIF/1211443-58-1.gif)
1211443-58-1
133 suppliers
$23.00/100mg

124-40-3
549 suppliers
$18.00/100ml
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
197 suppliers
$18.00/250mg
![(2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidin-6-yl)Methanol](/CAS/20150408/GIF/1374639-77-6.gif)
1374639-77-6
116 suppliers
$28.00/1g

124-40-3
549 suppliers
$18.00/100ml
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
197 suppliers
$18.00/250mg
733039-20-8
281 suppliers
$6.00/1g
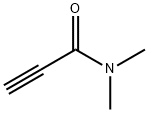
2682-34-0
15 suppliers
$1232.00/2.5g
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
197 suppliers
$18.00/250mg
![2-Chloro-7-cyclopentyl-6-methyl-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20180703/GIF/1178566-48-7.gif)
1178566-48-7
2 suppliers
inquiry

124-40-3
549 suppliers
$18.00/100ml
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
197 suppliers
$18.00/250mg