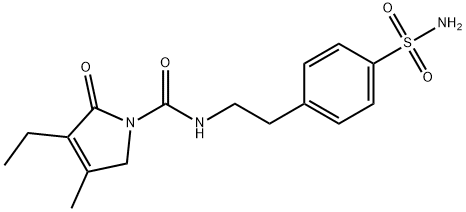
4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide synthesis
- Product Name:4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
- CAS Number:119018-29-0
- Molecular formula:C16H21N3O4S
- Molecular Weight:351.42
![4-{2-{[(3-Ethyl-2,5-dihydro-4-methyl-2-oxo-1H-pyrrol-1-yl)-carbonyl]-amino}-ethyl}-benzenesulfonyl chloride](/CAS/GIF/119043-16-2.gif)
119043-16-2
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
38.0 kg of methanol and 5.1 kg of industrial ammonia were sequentially added to a 100 L reactor and stirring was initiated. The crude wet product of Intermediate 2 was added slowly at 20-30°C and the reaction was maintained for 2 hours. The progress of the reaction was monitored by HPLC and after the reaction was complete, filtration was carried out and the filter cake was washed with deionized water. The obtained crude wet product of white Intermediate 3 was directly transferred to a 200 L reactor. Subsequently, 110.0 kg of methanol was added, the reaction temperature was raised and stirring was continued for 4 hours. After completion of the reaction, the mixture was cooled to 20-25 °C, the product was separated by filtration and dried to obtain 8.47 kg of white solid Intermediate 3 refined product, with purity >99% and interstitial isomer impurity content of 0.2% by HPLC.
![4-{2-{[(3-Ethyl-2,5-dihydro-4-methyl-2-oxo-1H-pyrrol-1-yl)-carbonyl]-amino}-ethyl}-benzenesulfonyl chloride](/CAS/GIF/119043-16-2.gif)
119043-16-2
23 suppliers
inquiry
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
262 suppliers
$17.00/1g
Yield:119018-29-0 97.8%
Reaction Conditions:
with ammonia in water at 80; for 1.5 h;
Steps:
1
120 g of chlorosulfonic acid was added to a three-necked bottle, and the compound m was added in portions. After the addition, the reaction was kept at 80 ° C for 0.5 hour. The reaction was completely monitored by TLC, and the temperature was lowered to 25 ° C. The reaction was dropped into ice water and filtered to obtain a solid. Adding solid to a solution of 200 ml of ammonia water and 100 ml of water, heating to 80 ° C for 1.5 hours, cooling to 30 ° C, adding hydrochloric acid to the reaction solution, separating the solid, filtering, washing with water, drying at 50 ° C, The obtained white solid was 25.2 g, and the purity by HPLC was 78.08%, the impurity V 7.24%, the impurity was 16.35%, and the yield was 97.8%.
References:
Weihai Disu Pharmaceutical Co., Ltd.;Disha Pharmaceutical Group Co., Ltd.;Wu Ronggui;Xue Fuzhao;Xu Keling;Wang Changde CN110092739, 2019, A Location in patent:Paragraph 0022
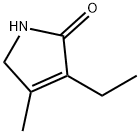
766-36-9
331 suppliers
$8.00/5g
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
262 suppliers
$17.00/1g
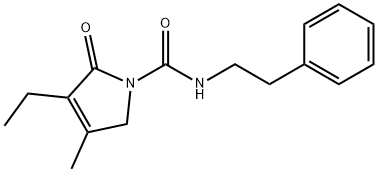
247098-18-6
65 suppliers
inquiry
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
262 suppliers
$17.00/1g
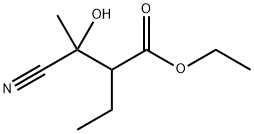
247098-17-5
14 suppliers
inquiry
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
262 suppliers
$17.00/1g
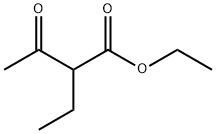
607-97-6
216 suppliers
$10.00/1g
![4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide](/CAS/GIF/119018-29-0.gif)
119018-29-0
262 suppliers
$17.00/1g