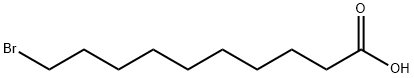
10-Bromodecanoic acid synthesis
- Product Name:10-Bromodecanoic acid
- CAS Number:50530-12-6
- Molecular formula:C10H19BrO2
- Molecular Weight:251.16

53463-68-6

50530-12-6
The general procedure for the synthesis of 10-bromodecanoic acid from 10-bromo-1-decanol is as follows: 1. 48% hydrobromic acid (22.6 mL, 0.2 mol, 1 eq.) was slowly added dropwise to a toluene solution (400 mL) of 1,10-decanediol (34.8 g, 0.2 mol, 1 eq.) with stirring and refluxed for 24 hr. at 180° C. using a Dean-Stark manifold. 2. After completion of the reaction, the mixture was cooled to room temperature and washed sequentially with 6N NaOH (150 mL), 10% HCl (150 mL), water (2 x 250 mL) and brine (200 mL). 3. The organic layer was dried over anhydrous sodium sulfate and concentrated, separated by silica gel column chromatography using cyclohexane/ethyl acetate (4:1) as eluent to afford 43.5 g (92%) of 10-bromo-1-decanol as a colorless liquid. 4. To an acetone solution (130 mL) of 10-bromo-1-decanol (41 g, 0.17 mol, 1 eq.) was slowly added a chromic acid solution prepared from chromium trioxide (25.7 g, 0.26 mol, 1.5 eq.), water (25 mL), and concentrated sulfuric acid (22.5 mL, 0.34 mol, 2 eq.) at -5°C. 5. The reaction mixture was stirred at 0°C for 2 hours and then left at room temperature overnight. 6. After completion of the reaction, the mixture was extracted with ether (3 x 250 mL), washed sequentially with water (250 mL) and brine (250 mL), dried over anhydrous sodium sulfate and concentrated. 7. The residue was separated by silica gel column chromatography using dichloromethane as eluent and recrystallized from petroleum ether to give 31.0 g (73%) of 10-bromodecanoic acid as a white solid with a melting point of 36-37 °C. The residue was separated by 1H2O2 column chromatography using dichloromethane as eluent. 8. The structure of the product was confirmed by 1H NMR and 13C NMR: 1H NMR δ 11.23 (bs, 1H, -OH), 3.41 (t, J = 7.0 Hz, 2H, H-10), 2.36 (t, J = 7.6 Hz, 2H, H-2), 1.87 (m, 2H, H-9), 1.64 (m, 2H, H-3), 1.22- 1.44 (m, 10H, H-3), 1.22- 2H, H-3). 1.44 (m, 10H, H-4-8); 13C NMR δ 180.2 (C1), 34.1 (C2), 34.0 (C10), 32.9, 29.1, 28.9, 28.6, 28.4, 28.2, 24.7.

53463-68-6
210 suppliers
$6.00/1g

50530-12-6
201 suppliers
$8.00/1g
Yield:50530-12-6 73%
Reaction Conditions:
with chromium(VI) oxide;sulfuric acid;water in acetone at -5 - 20;
Steps:
3.2.3. Synthesis of 10-bromodecanoic acid (4d)
To a solution of 1,10-decandiol (3) (34.8 g, 0.2 mol, 1 equiv) in toluene (400 mL) was added 48% HBr (22.6 mL, 0.2 mol, 1 equiv) dropwise with stirring and refluxed at 180 °C using Dean-Stark trap for 24 h. The mixture was cooled to room temperature and washed with 6 N NaOH (150 mL), 10% HCl (150 mL), H2O (2 x 250 mL) and brine (200 mL). The organic layer was dried over Na2SO4, concentrated and chromatographed on silica gel eluting with cyclohexane/ethylacetate (4:1) to give 43.5 g (92%) of 10-bromo-1-decanol as a colourless liquid. To a solution of 10-bromo-1-decanol (41 g, 0.17 mol, 1 equiv) in 130 mL of acetone at -5 °C was added slowly chromic acid solution prepared from CrO3 (25.7 g, 0.26 mol, 1.5 equiv), water (25 mL) and conc H2SO4 (22.5 mL, 0.34 mol, 2 equiv) at 0 °C, then stirred for 2 h and left over night at room temperature. The mixture was extracted with diethyl ether (3 x 250 mL), washed with water (250 mL) and brine (250 mL), dried over Na2SO4 and concentrated. The residue was chromatographed on silica gel eluting with CH2Cl2 afforded 31.0 g of 10-bromodecanoic acid (4d) (73%) as a white solid after recrystallization from petroleum ether. Mp 36-37 °C. 1H NMR δ 11.23 (bs, 1H, -OH), 3.41 (t, J = 7.0 Hz, 2H, H-10), 2.36 (t, J = 7.6 Hz, 2H, H-2), 1.87 (m, 2H, H-9), 1.64 (m, 2H, H-3), 1.22-1.44 (m, 10H, H-4-8). 13C NMR δ 180.2 (C1), 34.1 (C2), 34.0 (C10), 32.9, 29.1, 28.9, 28.6, 28.4, 28.2, 24.7.
References:
Wube, Abraham A.;Hüfner, Antje;Thomaschitz, Christina;Blunder, Martina;Kollroser, Manfred;Bauer, Rudolf;Bucar, Franz [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 1,p. 567 - 579] Location in patent:experimental part
14436-32-9
160 suppliers
$19.00/1g

50530-12-6
201 suppliers
$8.00/1g

7766-50-9
145 suppliers
$8.00/1g

50530-12-6
201 suppliers
$8.00/1g
![2-[(10-bromodecyl)oxy]tetrahydro-2H-pyran](/CAS/GIF/51795-88-1.gif)
51795-88-1
3 suppliers
inquiry

50530-12-6
201 suppliers
$8.00/1g
1679-53-4
246 suppliers
$10.00/1g

50530-12-6
201 suppliers
$8.00/1g