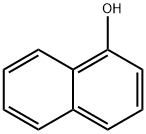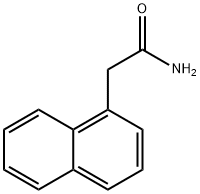
1-NAPHTHALENEACETAMIDE synthesis
- Product Name:1-NAPHTHALENEACETAMIDE
- CAS Number:86-86-2
- Molecular formula:C12H11NO
- Molecular Weight:185.22
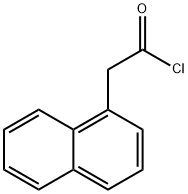
5121-00-6

86-86-2
The general procedure for the synthesis of 1-naphthaleneacetamide from naphthalen-1-ylacetyl chloride was as follows: 1-naphthaleneacetic acid (93.5 mg, 0.50 mmol) was dissolved in dichloromethane (0.8 mL), a catalytic amount of DMF was added, followed by the slow, dropwise addition of oxalyl chloride (135.6 mg, 1.00 mmol, 2.00 equiv.) under stirring. The reaction mixture was stirred at room temperature for 15 minutes. Upon completion of the reaction, the solution was concentrated under vacuum and the resulting residue was dissolved in tetrahydrofuran (0.8 mL). Ammonia (28%, 450.4 mg, 7.50 mmol, 15.0 eq.) was added to this solution at 0 °C, after which the reaction mixture was transferred to room temperature and stirred for 10 min. At the end of the reaction, the solution was concentrated in vacuum and purified by silica gel column chromatography (eluent: chloroform/methanol, 10/1, Rf = 0.50) to afford the target product 1-naphthaleneacetamide (Y-015) as a white solid in 72% yield.

86-87-3
568 suppliers
$13.50/25G

86-86-2
329 suppliers
$9.00/1g
Yield:86-86-2 98%
Reaction Conditions:
Stage #1:naphth-1-yl acetic acid with 1,3,5-trichloro-2,4,6-triazine;potassium carbonate in tetrahydrofuran for 0.0166667 h;Milling;
Stage #2: with ammonium thiocyanate in tetrahydrofuran for 0.0833333 h;Milling;
Steps:
2.General procedure for solvent-free reduction of carboxylic acid
Unless otherwise specified, carboxylic acid (0.542 mmol), TCT (0.0400 g, 0.216 mmol) and K2CO3 (0.2247 g, 1.626 mmol) were mixed together and hand ground for one minute using porcelain mortar and pestle. After addition of ammonium thiocyanate (0.0495 g, 0.650 mmol), the mixture was ground manually for further five minute. During the grinding, THF (calculated to be less than 1 L/mg of solids) was added to aid homogeneous mixing. The crude material was then purified by short column chromatography (column diameter 1.5 cm, packed with 3-4 g silica gel ) using 40-50% ethyl acetate/hexane as an eluent.
References:
Jaita, Subin;Phakhodee, Wong;Chairungsi, Neeranuch;Pattarawarapan, Mookda [Tetrahedron Letters,2018,vol. 59,# 39,p. 3571 - 3573] Location in patent:supporting information

5121-00-6
66 suppliers
$36.00/250 mg

86-86-2
329 suppliers
$9.00/1g
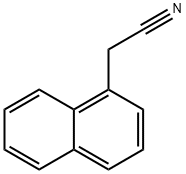
132-75-2
289 suppliers
$6.00/5g

86-86-2
329 suppliers
$9.00/1g
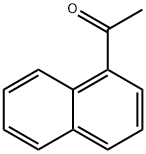
941-98-0
379 suppliers
$5.00/10g

86-86-2
329 suppliers
$9.00/1g