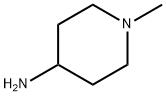
1-Methylpiperidin-4-amine synthesis
- Product Name:1-Methylpiperidin-4-amine
- CAS Number:41838-46-4
- Molecular formula:C6H14N2
- Molecular Weight:114.19
![Benzeneacetamide, 4-chloro-N-[(4-methylphenyl)methyl]-N-4-piperidinyl-](/CAS/20210305/GIF/359878-09-4.gif)
359878-09-4

41838-46-4
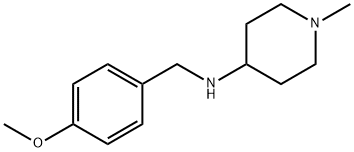
N-((4-methoxyphenyl)methyl)-4-amino-1-methylpiperidine (1 g, 4.27 mmol) was used as starting material, which was dissolved in a methanol solution of 4% formic acid (60 mL). Under argon protection, 10% Pd/C (1 g) was added to the reaction system and the reaction mixture was subsequently heated to reflux for 24 hours. Upon completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was acidified with concentrated hydrochloric acid to a pH of about 1. The resulting solution was concentrated to give a yellow oily substance. The oily substance was purified by fast chromatography (eluent: methanol/dichloromethane = 3:7 with 3.5% ammonia) to give 249 mg (51% yield) of 4-amino-1-methylpiperidine (57-MBT36B) as a white solid. Thin layer chromatography (TLC) Rf value was 0.13 (unfolding agent: 10% dichloromethane solution in methanol with 3.5% ammonia). High performance liquid chromatography-mass spectrometry (HPLC-MS, Method B) showed the molecular ion peak MH+ = 115. ultraviolet/mass spectrometry (UV/MS) detection showed a percent purity of -/100.
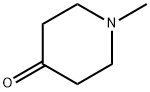
1445-73-4
446 suppliers
$15.00/25g

41838-46-4
258 suppliers
$6.00/1g
Yield:41838-46-4 100%
Reaction Conditions:
with ammonium formate;palladium on carbon in methanol;
Steps:
25.3A.1
Step: 3A-1Synthesis of l-Methyl-piperidin-4-yIamineProcedure:Ammonium formate (2.226g, 0.035348mol) was added to a solution of 1 -Methyl- piperidin-4-one (lg, 0.008837mol) in MeOH and stirred for lOmins. 20% Pd-C was added to this and the reaction mixture was heated at 60°C for 2hrs. The reaction was monitored by the TLC (10% methanol in chloroform). The reaction mixture was filtered through celite bed, concentrated to afford lg (100% yield) of l-Methyl-piperidin-4-ylamine.
References:
WO2012/59932,2012,A1 Location in patent:Page/Page column 137
![Benzeneacetamide, 4-chloro-N-[(4-methylphenyl)methyl]-N-4-piperidinyl-](/CAS/20210305/GIF/359878-09-4.gif)
359878-09-4
0 suppliers
inquiry

41838-46-4
258 suppliers
$6.00/1g
359878-55-0
8 suppliers
inquiry

41838-46-4
258 suppliers
$6.00/1g

34737-83-2
35 suppliers
inquiry

41838-46-4
258 suppliers
$6.00/1g

65220-86-2
31 suppliers
$157.00/250mg

41838-46-4
258 suppliers
$6.00/1g