
1-METHYL-1H-INDAZOLE-3-CARBALDEHYDE synthesis
- Product Name:1-METHYL-1H-INDAZOLE-3-CARBALDEHYDE
- CAS Number:4002-83-9
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
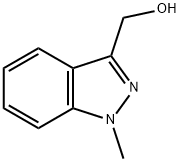
1578-96-7

4002-83-9
b) Synthesis of 1-methyl-1H-indazole-3-carboxaldehyde: 1-methyl-1H-indazole-3-methanol (0.320 g, 1.97 mmol, prepared from step a) was dissolved in 25 mL of dichloromethane (DCM), followed by the addition of Dess-Martin periodontane (0.920 g, 2.17 mmol). The reaction mixture was stirred at room temperature for 30 minutes. Upon completion of the reaction, 150 mL of diethyl ether was added to dilute, and 50 mL of 2 M sodium hydroxide solution was added to hydrolyze the suspension, and stirring was continued for 10 min. The organic layer was separated, washed sequentially with 1 M sodium hydroxide solution and water, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by a pre-populated silica gel column (Isolute, 10 g) with the eluent dichloromethane: methanol (98:2, v/v). The target product 1-methyl-1H-indazole-3-carbaldehyde was obtained in a yield of 0.271 g and 86%. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 10.21 (s, 1H), 8.29 (m, 1H), 7.50-7.43 (m, 2H), 7.36 (m, 1H), 4.18 (s, 3H).

1578-96-7
62 suppliers
$30.00/100mg

4002-83-9
83 suppliers
$56.00/250mg
Yield: 86%
Reaction Conditions:
Stage #1:(1-methyl-1H-indazol-3-yl)methanol with Dess-Martin periodane in dichloromethane for 0.5 h;
Stage #2: with sodium hydroxide;water in diethyl ether;dichloromethane for 0.166667 h;
Steps:
b
b) 1-Methyl-lH-indazole-3-carbaldehyde; (1-Methyl-lH-indazol-3-yl) methanol (0.320 g, 1.97 mmol, from step a above) was dissolved in 25 mL of DCM and Dess-Martin periodinane (0.920 g, 2.17 mmol) was added. The mixture was stirred for 30 min after which 150 mL of diethyl ether was added and the suspension was hydrolyse by addition of 50 ml of 2M NaOH and stirring for 10 min. The ether layer was washed with 1M NaOH and water, dried over Na2SO4, filtered and evaporated. The crude product was chromatographed on a pre-packed Si02-column (Isolute, 10 g) eluted with DCM: MeOH 98: 2. Yield: 0.271 g (86%). 'H NMR (300 MHz, CDC13) 8 10.21 (s, 1H), 8.29 (m, 1H), 7.50-7. 43 (m, 2H), 7.36 (m, 1H), 4.18 (s, 3H).
References:
ASTRAZENECA AB WO2005/66132, 2005, A1 Location in patent:Page/Page column 45-46
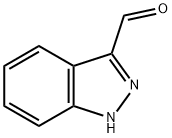
5235-10-9
140 suppliers
$15.00/1g

74-88-4
357 suppliers
$15.00/10g

4002-83-9
83 suppliers
$56.00/250mg
50890-83-0
424 suppliers
$10.00/1g

4002-83-9
83 suppliers
$56.00/250mg