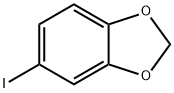
1-IODO-3,4-METHYLENEDIOXYBENZENE synthesis
- Product Name:1-IODO-3,4-METHYLENEDIOXYBENZENE
- CAS Number:5876-51-7
- Molecular formula:C7H5IO2
- Molecular Weight:248.02
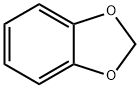
274-09-9

5876-51-7
N-iodosuccinimide (14.8 g, 66.0 mmol), 1,3-benzodioxole (6.31 mL, 55.0 mmol) and acetic acid (470 mL) were added to a 1 L flask under argon protection. The reaction mixture was stirred at room temperature for 65 hours. Upon completion of the reaction, the acetic acid was removed by distillation under reduced pressure and the residue was neutralized with saturated aqueous sodium bicarbonate solution. Subsequently, aqueous sodium thiosulfate and chloroform were added to the reaction mixture to separate the organic layer. The aqueous layer was further extracted with chloroform. All organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using hexane as eluent to afford 5-iodo-1,3-benzodioxole (11.3 g, 45.7 mmol, 83% yield) in colorless liquid form.

274-09-9
479 suppliers
$5.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5876-51-7
119 suppliers
$57.00/250mg
Yield: 57%
Reaction Conditions:
in acetic acid
Steps:
37 3-Benzyl-1,5-epoxy-7,8methylenedioxy-1,2,4,5-tetrahydro-3-benzazepin oxalate
EXAMPLE 37 3-Benzyl-1,5-epoxy-7,8methylenedioxy-1,2,4,5-tetrahydro-3-benzazepin oxalate To a solution of 1,2-methylenedioxybenzene (24.4 g, 0.20M) in AcOH (30 ml) was added ICI (40 g, 0.250M) in AcOH (50 ml) at room temperature over 0.5 hour. The reaction mixture was stirred for an additional three hours, then poured into H2 O and extracted twice with ether. The organic extracts were washed with NaHSO3, saturated NaHCO3, H2 O, dried (MgSO4), and evaporated in vacuo. The product was distilled under vacuum at 70` C. to give 3,4-methylenedioxyiodobenzene as a pale orange liquid (28.8 g, 57% yield). NMR (CDCl3) δ 5.85 (s, 2H, O--CH2 --O), 6.50 (d, 1H, J=8 Hz, H6), 7.10 (m, 2H, J=8 Hz, J=2 Hz, H2 and H5); MS: 248 (M+).
References:
Ortho Pharmaceutical Corporation US4761413, 1988, A

274-09-9
479 suppliers
$5.00/5g

5876-51-7
119 suppliers
$57.00/250mg
2635-13-4
303 suppliers
$5.00/1g

5876-51-7
119 suppliers
$57.00/250mg
14268-66-7
273 suppliers
$20.00/5 g

5876-51-7
119 suppliers
$57.00/250mg
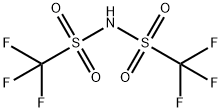
82113-65-3
257 suppliers
$25.00/1 g

14268-66-7
273 suppliers
$20.00/5 g

5876-51-7
119 suppliers
$57.00/250mg