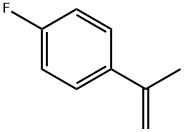
1-Fluoro-4-(1-methylethenyl)benzene synthesis
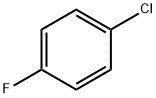
- Product Name:1-Fluoro-4-(1-methylethenyl)benzene
- CAS Number:350-40-3
- Molecular formula:C9H9F
- Molecular Weight:136.17
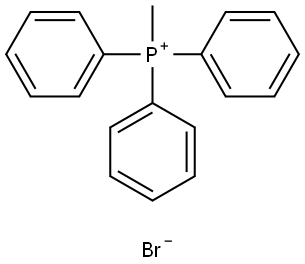
1779-49-3

403-42-9

350-40-3
GENERAL METHOD: Potassium tert-butoxide (t-BuOK, 2.47 g, 22.00 mmol) was added to an anhydrous tetrahydrofuran (THF, 70 mL) solution of methyltriphenylphosphonium bromide (7.12 g, 20.00 mmol) in four batches at 0 °C. The reaction mixture was stirred at 0 °C for 2 h, followed by slow dropwise addition of anhydrous THF (20 mL) solution of 4-fluoroacetophenone (20 mmol) over 30 min. After the dropwise addition, the reaction mixture was continued to be stirred at room temperature for 16 hours. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride (NH4Cl, 50 mL) solution. The reaction solution was extracted three times with petroleum ether (3 x 50 mL). All organic phases were combined, washed with saturated saline (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated. Finally, the residue was purified by fast column chromatography using petroleum ether (Pet) as eluent to afford the target product 1-fluoro-4-(prop-1-en-2-yl)benzene.

403-42-9
547 suppliers
$5.00/10g

350-40-3
137 suppliers
$8.00/1g
Yield: 100%
Reaction Conditions:
Stage #1:methyl-triphenylphosphonium iodide with potassium tert-butylate in tetrahydrofuran at 0; for 1 h;
Stage #2:1-(4-fluorophenyl)ethanone in tetrahydrofuran at 0 - 20; for 2 h;
Steps:
A.i
Potassium tert-butoxide (52.9 g, 0.47 mol) was added to the suspension of methyl triphenylphosphonium iodide (190 g, 0.47 mol) in THF (400 ml) at ice-cooled condition. After 1 h stirring at ice-cooled condition, a solution of 4-Fluoro acetophenone (30 g, 0.199 mol) in THF (100 ml) was added dropwise. Then the reaction mixture was stirred at room temperature for 2 h and quenched with sat. ammonium chloride solution. THF was removed under reduced pressure and the reaction mixture was extracted with petether, washed with water, brine and dried over anh. sodium sulphate nitrated. The sub-title compound (30 g, 100%) was obtained as pale yellow liquid by concentration of petroleum ether layer
References:
ASTRAZENECA AB US2008/15237, 2008, A1 Location in patent:Page/Page column 27

402-41-5
76 suppliers
$22.00/1g

350-40-3
137 suppliers
$8.00/1g