
1-Ethyl-3-methylimidazolium bromide synthesis
- Product Name:1-Ethyl-3-methylimidazolium bromide
- CAS Number:65039-08-9
- Molecular formula:C6H11BrN2
- Molecular Weight:191.07
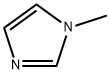
616-47-7
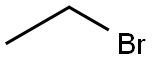
74-96-4

65039-08-9
All ligands (except #3) were synthesized with reference to the modified Starikova et al. method [20]. Typical generic synthesis steps are given below with spectral and analytical data. N-monosubstituted imidazole (0.1 mmol) and anhydrous toluene were added to a two-necked flask and stirred until a homogeneous solution was formed; followed by the slow dropwise addition of ethyl bromide (0.3 mmol) under continuous stirring. After the dropwise addition, the reaction mixture was heated with stirring at 40 °C for 24 hours. After completion of the reaction, the solvent was removed under reduced pressure and the resulting product was dried under vacuum to give the target ligand.2.2.2 1-Ethyl-3-methylimidazolium bromide (2) was a white solid with a yield of 4.70 g (98% yield).IR (ATR, cm?1): 3065, 2975, 1670, 1571, 1467, 1172, 1101, 856, 789, 649, 621, 417. 649, 621, 417; 1H NMR (400 MHz, CDCl3): δ 1.47 (3H, t, J = 7.3 Hz, CH3), 3.97 (3H, s, NCH3), 4.32 (2H, q, NCH2), 7.54 (2H, s, NCH), 10.07 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δ 15.64 (CH3), 36.63 (NCH3), 45.18 (NCH2), 122.01 (NCH), 123.71 (NCH), 136.73 (NCH). Mass spectrum (ESI): m/z 111.5 (M+?Br?). High-resolution mass spectrum (ESI): calculated value C6H11BrN2 (M+?Br?) 111.09222, measured value 111.09196.
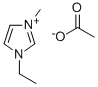
143314-17-4
225 suppliers
$6.00/1g

106-93-4
410 suppliers
$15.00/5g
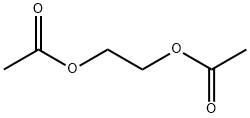
111-55-7
390 suppliers
$5.00/100g

65039-08-9
342 suppliers
$9.00/10g
Yield:111-55-7 90%
Reaction Conditions:
at 20; for 3 h;Inert atmosphere;
Steps:
2
Example 1 and 2: ethylene glycol diacetate; A 100 mL Schlenk flask was equipped with a magnetic stirring bar, EMIM-acetate (50.0 g, 0.293 mol), and 0.5 equivalents of 1,2-dichloroethane (14.5 g, 0.146 mol) were added at room temperature and reaction was stirred at 70°C for 3h. After reaction two phases were obtained upper layer was decanted and the reaction mixture was extracted 3 times with diethyl ether, all fractions were combined and diethyl ether was removed under vacuum. The final pure product was obtained as color less liquid (90.0% yield) When 1, 2-dibromoethane was used as substrate the reaction was very fast even at room temperature and was highly exothermic. The reaction proceeds via homogeneous pathway; the pure product was distilled from the reaction mixture at 100°C under reduced pressure (90.0% yield). Ethylene glycol diacetate: 1H NMR (CDCl3, 400.13 MHz): δ = 2.02 (s, 6 H, CH3), 4.2 (s, 4H, CH2); 13C NMR: δ = 20.9 (CH3), 62.3 (CH2), 170.9 (CO); FTIR (ATR mode): v = 2961 (br), 1735 (s), 1371 (m), 1213 (s), 1048 (m).
References:
BASF SE;Universit?t Siegen Institut für Anorganische Chemie EP2532646, 2012, A1 Location in patent:Page/Page column 5-7

616-47-7
764 suppliers
$10.00/100g

74-96-4
458 suppliers
$9.00/5g

65039-08-9
342 suppliers
$9.00/10g

143314-17-4
225 suppliers
$6.00/1g

74-95-3
272 suppliers
$10.00/10g
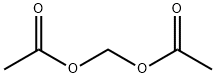
628-51-3
69 suppliers
$12.00/250mg

65039-08-9
342 suppliers
$9.00/10g

616-47-7
764 suppliers
$10.00/100g

105-58-8
479 suppliers
$14.00/25g

65039-08-9
342 suppliers
$9.00/10g

7098-07-9
337 suppliers
$5.00/10g
74-83-9
0 suppliers
$24.10/48624

65039-08-9
342 suppliers
$9.00/10g