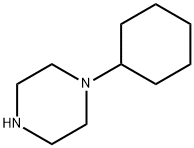
1-Cyclohexylpiperazine synthesis
- Product Name:1-Cyclohexylpiperazine
- CAS Number:17766-28-8
- Molecular formula:C10H20N2
- Molecular Weight:168.28

1224935-95-8

17766-28-8
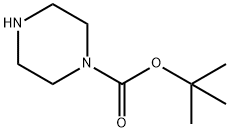
General procedure for the synthesis of 1-cyclohexylpiperazine from tert-butyl 4-cyclohexylpiperazine-1-carboxylate: tert-butyl 4-cyclohexylpiperazine-1-carboxylate (1.38 g, 5.15 mmol) was dissolved in a mixture of ethanol/hydrochloric acid (15 mL), and the reaction was stirred for 18 h at room temperature. After completion of the reaction, the solvent was removed by distillation under reduced pressure. To the residue, 5 mL of water was added and the aqueous phase was washed with ethyl acetate (5 mL × 3). Subsequently, the aqueous phase was adjusted to pH 7~8 with base and extracted with ethyl acetate (10 mL × 3). All organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give 0.78 g of 1-cyclohexylpiperazine (confirmed by LC-MS and TLC, yield: 90.1%).

1224935-95-8
37 suppliers
inquiry

17766-28-8
266 suppliers
$8.00/1g
Yield:17766-28-8 90.1%
Reaction Conditions:
with hydrogenchloride in ethanol;water at 20; for 18 h;
Steps:
A solution of comp A0023-2 (1.38 g, 5.15 mmol) in ethanol/hydrochloric acid (15 mL) was stirred overnight (about 18 hours) at room temperature. Then, the solvent was removed and 5 mL of H2O was added into the mixture. The mixture was washed with ethyl acetate (5 ml x 3) and the aqueous phase was neutralized to pH=7~8 and extracted with ethyl acetate (10 ml x 3) . The combined organic phase was dried and concentrated and to yield 0.78 g of the title product (LC-MS and TLC confirmed, yield: 90.1%) .
References:
WO2010/51374,2010,A1 Location in patent:Page/Page column 84; 85
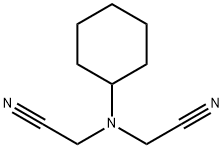
4823-13-6
0 suppliers
inquiry

17766-28-8
266 suppliers
$8.00/1g
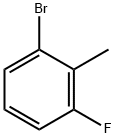
1422-54-4
236 suppliers
$5.00/1g

17766-28-8
266 suppliers
$8.00/1g
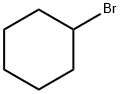
108-85-0
343 suppliers
$5.00/10g
57260-71-6
741 suppliers
$5.00/5g

17766-28-8
266 suppliers
$8.00/1g
