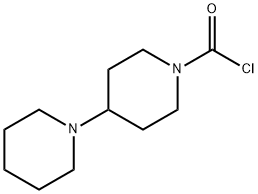
1-CHLOROCARBONYL-4-PIPERIDINOPIPERIDINE synthesis
- Product Name:1-CHLOROCARBONYL-4-PIPERIDINOPIPERIDINE
- CAS Number:103816-19-9
- Molecular formula:C11H19ClN2O
- Molecular Weight:230.73
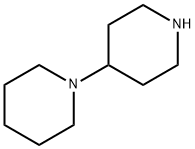
4897-50-1
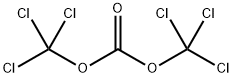
32315-10-9

103816-19-9
The general procedure for the synthesis of piperidinyl piperidine carbonyl chloride from 4-piperidinyl piperidine and triphosgene is as follows: Example 1 Synthesis of [1,4']bipiperidine-1'-carbonyl chloride (II): triphosgene (16.48 g) was dissolved in methylene chloride (200 ml), and methylene chloride solution of 4-piperidinyl piperidine (20 g) (200 ml) was slowly added, and the temperature of the reaction was maintained at 20 to 25 °C and the reaction time was 1-2 hours. During this process, dichloromethane was removed by distillation and acetonitrile was added subsequently. Distillation was continued to remove dichloromethane and acetonitrile until the reaction temperature was raised to 70°C. Fresh dichloromethane was added to the reaction mixture and after forming a homogeneous slurry, potassium carbonate (30-35 g) was added and the reaction mixture was stirred for 1-2 hours. After completion of the reaction, the reaction mixture was filtered and the filtrate was concentrated. Hexane was slowly added to the concentrate under stirring to precipitate a solid product. The solid was collected by filtration, washed with hexane and dried under reduced pressure at about 40 °C to give 27 g of product (98.5% yield). The product was analyzed by GC showing a purity of 99.80% and no dimer impurities were detected.

4897-50-1
384 suppliers
$19.00/5g

32315-10-9
438 suppliers
$10.00/1g

103816-19-9
79 suppliers
$43.00/1g
Yield: 99%
Reaction Conditions:
with triethylamine in dichloromethane at -10 - 20; for 4 h;
Steps:
5.1 Step 1) [1,4'-Bipiperidine] -1'-formyl chloride
Dissolve triphosgene (882mg, 2.97mmol) in 30mL of dichloromethane, and then dropwiseadd 1,4'-bipiperidine (500mg, 2.97mmol) and triethylamine (900mg, 8.91mmol)at -10 ° C.Dichloromethane solution (20 mL). After the dropwise addition was continued, the reaction was stirred at -10 ° C for 2 hours, and then transferredto room temperature for 2 hours.It was concentrated under reduced pressure, 20 mL of tetrahydrofuran was added to the residue, and the mother liquid was concentrated under reduced pressure to obtain the white title compound(680 mg, yield: 99%).
References:
Guangdong Dongyangguang Pharmaceutical Co., Ltd.;Wang Xiaojun;Zhou Pingjian;Yang Chuanwen;Zhang Yingjun CN104387358, 2016, B Location in patent:Paragraph 0161-0164

4897-50-1
384 suppliers
$19.00/5g
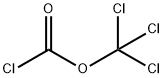
503-38-8
122 suppliers
$15.00/5g

103816-19-9
79 suppliers
$43.00/1g