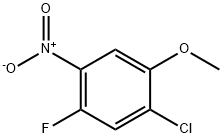
1-CHLORO-5-FLUORO-2-METHOXY-4-NITROBENZENE synthesis
- Product Name:1-CHLORO-5-FLUORO-2-METHOXY-4-NITROBENZENE
- CAS Number:84478-76-2
- Molecular formula:C7H5ClFNO3
- Molecular Weight:205.57
84478-75-1

74-88-4

84478-76-2
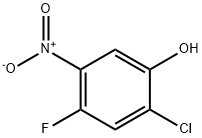
Potassium carbonate (18 g, 131 mmol) and iodomethane (8.0 mL, 131 mmol) were added to a solution of 2-chloro-4-fluoro-5-nitrophenol (5.0 g, 26.1 mmol) in acetone (100 mL) and stirred at room temperature. The reaction mixture was stirred at room temperature for 2 h. The progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the solvent was removed by evaporation under reduced pressure. The residue was diluted with distilled water (100 mL) and extracted with ethyl acetate (3 x 100 mL). The organic layers were combined, washed sequentially with distilled water (100 mL) and saturated saline (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give 2-chloro-4-fluoro-5-nitroanisole as a yellow solid. Yield: 5.0 g (93%). 1H NMR (400 MHz, DMSO-d6): δ 7.94-7.97 (m, 1H), 7.81-7.83 (m, 1H), 3.95 (s, 3H).

84478-75-1
194 suppliers
$10.00/1g

74-88-4
358 suppliers
$15.00/10g

84478-76-2
119 suppliers
$5.00/100mg
Yield: 93%
Reaction Conditions:
with potassium carbonate in acetone at 20; for 2 h;
Steps:
1 -Chloro-5-tI uoro-2-methoxy-4-nitrobenzene
To a solution of 2-chloro-4-fluoro-5-nitrophenol (5.Og, 26.lmmol) in acetone (lOOmL) were added K2003 (18 g, l3lmmol) and methyl iodide (8.OmL, 131 mmol) at rt. The reaction mixture was stirred at rt for 2h. TLC showed the reaction to be complete. The reaction mixture was evaporated under reduced pressure, diluted with H20 (lOOmL) and extracted with EtOAc (3xlOOmL). The organic layer was washed with H20 (lOOmL), brine (lOOmL), dried (Na2SO4), filtered and concentrated under reduced pressure to give 1 -chloro-5-fluoro-2-methoxy-4-nitrobenzene as a yellow solid. Yield: 5.0 g (93%); 1H NMR (400 MHz, DMSO-d6): 7.94-7.97 (m, 1H), 7.81-7.83 (m, 1H) 3.95 (5, 3H).
References:
DISCUVA LTD.;MEO, Dr Paul;KHAN, Dr. Nawaz WO2018/37223, 2018, A1 Location in patent:Page/Page column 206; 207