
1-(Bromomethyl)naphthalene synthesis
- Product Name:1-(Bromomethyl)naphthalene
- CAS Number:3163-27-7
- Molecular formula:C11H9Br
- Molecular Weight:221.09
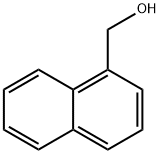
4780-79-4

3163-27-7
The general procedure for the synthesis of 1-bromomethylnaphthalene from 1-naphthalenemethanol: Ethyl α,α-diacetoacetate (0.41 mmol, 1.2 eq.), 1-naphthalenemethanol (0.34 mmol, 1.0 eq.), and triphenylphosphine (0.68 mmol, 2.0 eq.) were added to 3 mL of dichloroethane (DCE) at room temperature and the reaction took place in air. The progress of the reaction was monitored by thin layer chromatography (TLC) during the reaction. Upon completion of the reaction, the reaction was quenched by the addition of 3 mL of water, followed by extraction with ethyl acetate (3 x 3 mL). The organic phases were combined, washed with saturated saline, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography, and the eluent was petroleum ether or a mixed solvent of petroleum ether and ethyl acetate, and the target product 1-bromomethyl naphthalene was finally obtained.

4780-79-4
246 suppliers
$6.00/5g

3163-27-7
195 suppliers
$7.00/1g
Yield:3163-27-7 99%
Reaction Conditions:
with ethyl 2,2-dibromoacetoacetate;triphenylphosphine in dichloromethane at 20; for 0.5 h;
Steps:
General Procedure for the Synthesis of products
General procedure: Ethyl α,α-dibromoacetoacetate 2a (0.41 mmol, 1.2 equiv), alcohols 1a-1s (0.34 mmol, 1.0 equiv) and Ph3P (0.68 mmol, 2.0 equiv) were added under ambient temperature to 3 mL of DCE in air. After stirred at room temperature for appropriate time (monitored by TLC), the reaction was quenched by addition of H2O (3 mL) and then extracted with ethyl acetate (3×3 mL). The combined organic layer was washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by column chromatography on silica gel with petroleum ether or mixture of petroleum ether and ethyl acetate as eluent to afford the corresponding products 3a-3s.
References:
Cui, Xiao-Meng;Guan, Yong-Hong;Li, Na;Lv, Hao;Fu, Lin-An;Guo, Kun;Fan, Xiaohui [Tetrahedron Letters,2014,vol. 55,# 1,p. 90 - 93] Location in patent:supporting information

66-77-3
386 suppliers
$7.00/10g

3163-27-7
195 suppliers
$7.00/1g
90-12-0
294 suppliers
$17.00/25mL

3163-27-7
195 suppliers
$7.00/1g

33250-32-7
10 suppliers
inquiry

3163-27-7
195 suppliers
$7.00/1g

90-12-0
294 suppliers
$17.00/25mL
6627-78-7
115 suppliers
$13.00/1g

3163-27-7
195 suppliers
$7.00/1g