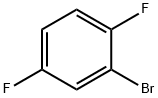
1-Bromo-2,5-difluorobenzene synthesis
- Product Name:1-Bromo-2,5-difluorobenzene
- CAS Number:399-94-0
- Molecular formula:C6H3BrF2
- Molecular Weight:192.99
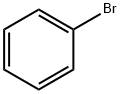
108-86-1
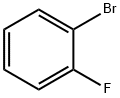
1072-85-1

399-94-0
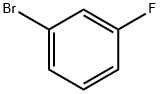
1073-06-9
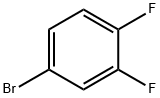
348-61-8

460-00-4
GENERAL STEPS: In a FEP or PFA reactor equipped with a PTFE-lined magnetic stir bar and connected to a gas scrubber bottle, substituted benzene (0.95-1.10 mmol), 1,1,1,3,3-pentafluorobutane (2 mL per mL of C6H5R), and BF3-Et2O (1.3-1.5 mmol/mmol C6H5R) were added. The mixture was stirred at 0-5 °C (ice bath) for 10-15 min, followed by batchwise addition of XeF2 (1.2-1.3 mmol/mmol C6H5R). After each addition, the mixture was stirred at 22-25 °C for 3-5 min and cooled to 0-5 °C again. After the addition was completed, the dark-colored solution was continued to be stirred at 22-25 °C for 15-30 min. Upon completion of the reaction, the reaction was quenched by the addition of 10% aqueous KHCO3, the upper organic layer was separated and filtered through a short column packed with silica gel (40-60 μm) and finally dried with magnesium sulfate. The solution was analyzed by 19F NMR and GC/MS. The major products are listed in the table and the others are as follows (GC/MS data provided).
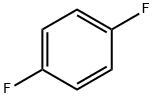
540-36-3
295 suppliers
$10.00/5g

399-94-0
390 suppliers
$6.00/5g
Yield:399-94-0 92.7%
Reaction Conditions:
with N-Bromosuccinimide;sulfuric acid at 30 - 40; for 1 h;Large scale;
Steps:
1.1; 2.1 (1) Bromination reaction:
Add 1000 g of p-difluorobenzene to the reaction vessel, 5000 mL of sulfuric acid, controlled to 30 °C in a water bath. 1620 g of N-bromosuccinimide was slowly added with stirring. Do not exceed 40 °C during the addition. After the addition, the reaction was incubated for 1 h and the GC was followed until the reaction was over. After the reaction, the reaction product was poured into ice water to precipitate a solid, which was suction filtered. 1568 g of 2,5-difluorobromobenzene was obtained as a pale yellow powdery solid, the content was 98%, and the yield was 92.7%.
References:
CN109369412,2019,A Location in patent:Paragraph 0009; 0013

108-86-1
525 suppliers
$10.00/5g

1072-85-1
460 suppliers
$5.00/10g

399-94-0
390 suppliers
$6.00/5g

1073-06-9
448 suppliers
$6.00/10g

348-61-8
396 suppliers
$6.00/5g

460-00-4
636 suppliers
$6.00/10g

540-36-3
295 suppliers
$10.00/5g

399-94-0
390 suppliers
$6.00/5g
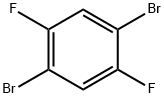
327-51-5
286 suppliers
$5.00/5g
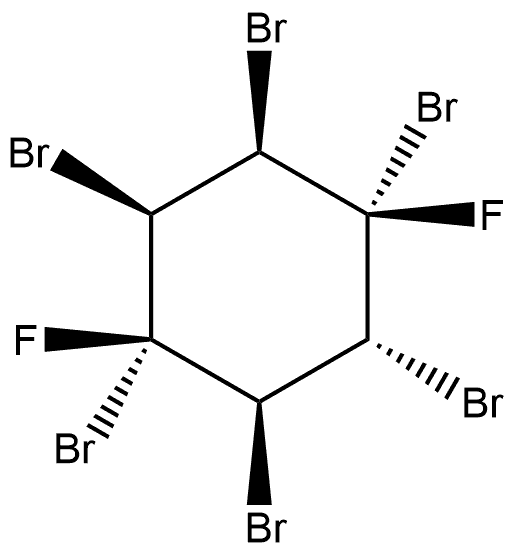
128259-70-1
0 suppliers
inquiry
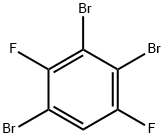
128259-69-8
3 suppliers
inquiry