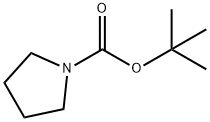
1-Boc-Pyrrolidine synthesis
- Product Name:1-Boc-Pyrrolidine
- CAS Number:86953-79-9
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
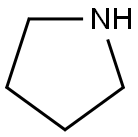
123-75-1

24424-99-5

86953-79-9
The general procedure for the synthesis of 1-Boc-tetrandrine from tetrandrine and di-tert-butyl dicarbonate is as follows: the synthesis was carried out by a modified literature method (omitting the Sparteine chiral ligand), as specified in J. Am. Chem. Soc. 1994, 116, 3231.The steps are as follows: A) Preparation of Boc-protected pyrrolidines Triethylamine (TEA, 15.6 g, 0.15 mol) was slowly added to a dichloromethane (DCM, 150 mL) solution of pyrrolidine (10.0 g, 0.14 mol) in stirring at 0 °C. Subsequently, di-tert-butyl dicarbonate ((Boc)2O, 30.6 g, 0.14 mol) was added, and the reaction mixture was stirred at room temperature for 1 hour. The progress of the reaction was monitored by thin layer chromatography (TLC) to confirm complete pyrrolidine consumption. Upon completion of the reaction, the reaction mixture was washed sequentially with 1 M aqueous hydrochloric acid (100 mL) and brine, and the organic phase was dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure to afford the colorless, oily product 1-Boc-tetrandrine (24.0 g, 100%), which could be used in the subsequent steps without further purification.

123-75-1
586 suppliers
$10.00/5g

24424-99-5
865 suppliers
$13.50/25G

86953-79-9
187 suppliers
$9.00/1g
Yield:86953-79-9 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1 h;
Steps:
19.1.A Example19: Compound 61 (3'S,5'S)-2-benzyl-l'-(2,2-diphenylacetyl)-[l,3'- bipyrrolidine]-5'-carboxylic acid 1. Procedure for the preparation of2-benzyl pyrrolidine.
Racemic 2-benzyl pyrrolidine was synthesized using a modified literature procedure (the Sparteine chiral ligand was omitted), see: J. Am. Chem. Soc. 1994, 116, 3231 as follows: A) Procedure for the preparation of Boc-protected pyrrolidine To a stirred mixture of pyrrolidine (10.0 g, 0.14 mol) in DCM (150 mL) at 0°C was added TEA (15.6 g, 0.15 mol) followed by (Boc)20 (30.6 g, 0.14 mol) and the mixture was stirred at RT for 1 h, TLC showed that pyrrolidine had disappeared. The mixture was washed with a 1 M aqueous HC1 solution (100 mL), brine, dried over Na2S04, filtered and concentrated in vacuo to give the product (24.0 g, 100%) as a colorless oil, which was used in the next step directly.
References:
SPINIFEX PHARMACEUTICALS PTY LTD;MCCARTHY, Thomas David;NAYLOR, Alan WO2013/102242, 2013, A1 Location in patent:Page/Page column 94; 95
5176-27-2
184 suppliers
$10.00/1g

86953-79-9
187 suppliers
$9.00/1g
15761-39-4
618 suppliers
$5.00/10g

86953-79-9
187 suppliers
$9.00/1g
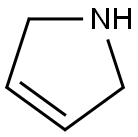
109-96-6
208 suppliers
$16.00/500mg

24424-99-5
865 suppliers
$13.50/25G
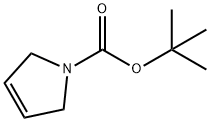
73286-70-1
289 suppliers
$6.00/5g

86953-79-9
187 suppliers
$9.00/1g
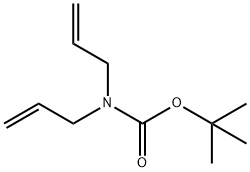
151259-38-0
52 suppliers
$49.00/10g

86953-79-9
187 suppliers
$9.00/1g