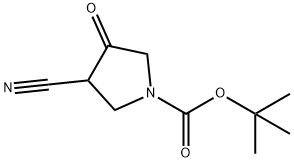
1-Boc-3-cyano-4-oxopyrrolidine synthesis
- Product Name:1-Boc-3-cyano-4-oxopyrrolidine
- CAS Number:175463-32-8
- Molecular formula:C10H14N2O3
- Molecular Weight:210.23
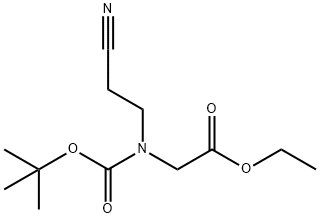
266353-18-8

175463-32-8
General procedure for the synthesis of 1-Boc-3-cyano-4-pyrrolidinone from ethyl 2-(BOC-(2-cyanoethyl)amino)acetate: 2-(BOC-(2-cyanoethyl)amino)ethyl acetate (16.5 g), an oil, was dissolved in 50 mL of anhydrous ethanol. The solution was slowly added dropwise to 100 mL of anhydrous ethanol solution containing 4 g (0.17 mol) of sodium metal under heated reflux conditions and the reaction was continued at reflux for 1 hour. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in 100 mL of water and the aqueous layer was subsequently washed with ethyl acetate (30 mL x 2). The pH of the aqueous layer was adjusted to 4 with 1 mol/L hydrochloric acid and extracted with ethyl acetate. The organic phase was dried with anhydrous magnesium sulfate and filtered. The filtrate was concentrated to about 20 mL, filtered, and the filter cake was washed with a small amount of ethyl acetate and dried to give 16.5 g of white solid 1-Boc-3-cyano-4-pyrrolidinone in 68% yield.

266353-18-8
13 suppliers
inquiry

175463-32-8
244 suppliers
$6.00/1g
Yield:175463-32-8 68%
Reaction Conditions:
with sodium in ethanol for 1 h;Reflux;
Steps:
1 Synthesis of 4-cyano-1- (N-tert-butoxycarbonyl) pyrrolidin-3-one (3)
The oil 2 was dissolved in 50 mL of absolute ethanol,The solution was added dropwise to a solution of 4 g (0.17 mol) of metallic sodium in 100 mL of absolute ethanol under reflux with heating,Refluxed for 1 h.The solvent was distilled off under reduced pressure, and the residue was treated with 100 mLWater was dissolved and washed with ethyl acetate (30 mL * 2). The aqueous layer was adjusted to pH 4 with 1 mol / L hydrochloric acid, extracted with ethyl acetate,Dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated to a residual solvent of about 20 mL, filtered and the cake washed with a small amount of ethyl acetateDry cake to give 16.5 g of a white solid. Yield: 68%.
References:
Huaren Pharmaceutical Co Ltd;Guo, Jin;Feng, Xinguang;Han, Yong;Jiang, Ming;Li, Jiren;Zou, Shanshan CN105585518, 2016, A Location in patent:Paragraph 0024; 0026
![N-(2-Cyanoethyl)-N-[(1,1-dimethylethoxy)carbonyl]glycine methyl ester](/CAS/20180702/GIF/266353-19-9.gif)
266353-19-9
9 suppliers
inquiry

175463-32-8
244 suppliers
$6.00/1g
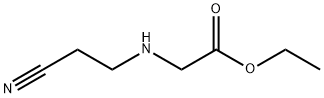
44981-94-4
32 suppliers
inquiry

175463-32-8
244 suppliers
$6.00/1g

3088-42-4
83 suppliers
$17.67/250mgs:

175463-32-8
244 suppliers
$6.00/1g

24424-99-5
867 suppliers
$13.50/25G

175463-32-8
244 suppliers
$6.00/1g