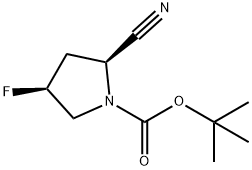
1-Boc-(2S,4S)-2-cyano-4-fluoropyrrolidine synthesis
- Product Name:1-Boc-(2S,4S)-2-cyano-4-fluoropyrrolidine
- CAS Number:426844-76-0
- Molecular formula:C10H15FN2O2
- Molecular Weight:214.24
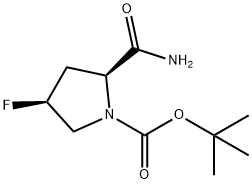
426844-22-6

426844-76-0
Step 2: Synthesis of (2S,4S)-1-Boc-2-cyano-4-fluoropyrrolidine To a stirred and cooled to 0 °C solution of (2S,4S)-N-Boc-4-fluoropyrrolidine-2-carboxamide (10 g, 43.10 mmol) in anhydrous THF (50 ml) was sequentially added triethylamine (13.93 g, 138 mmol) and trifluoroacetic anhydride (14.5 g, 69.05 mmol). The reaction mixture was continued to be stirred at 0°C for 1 hour. After completion of the reaction, the reaction was quenched with 100 ml of water and extracted with chloroform (2 x 100 ml). The organic phases were combined, washed sequentially with water (2 x 100 ml) and brine (50 ml) and dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure to give 9.0 g of off-white solid product in 97.6% yield. IR (KBr, cm-1): 2979, 2243, 1387, 1240, 1168, 1123, 1072, 960. 1H NMR (CDCl3, 300MHz) δ: 1.49-1.53(d, rotary isomer, 9H), 2.25-2.47(m, 1H), 2.64(t, J=14.7Hz, 1H), 3.52(dd, J=9.6,3.6Hz, 0.5H, rotary isomer), 3.64(dd, J=9.3,3.3Hz , 0.5H, rotary isomer), 3.73-3.94 (m, 1H), 4.64 (dd, J=8.7 Hz, 0.6H, rotary isomer), 4.76 (dd, J=8.7 Hz, 0.4H, rotary isomer), 5.31 (brd, J=51.3 Hz, 1H).
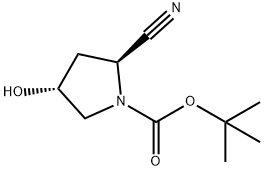
483366-12-7
70 suppliers
inquiry

426844-76-0
106 suppliers
inquiry
Yield:426844-76-0 100%
Reaction Conditions:
Stage #1:(2S,4R)-N-Boc-2-cyano-4-hydroxypyrrolidine with diethylamino-sulfur trifluoride in dichloromethane at -30 - 20;Inert atmosphere;
Stage #2: with sodium carbonate in dichloromethane;water; pH=7 at 20;
Steps:
13.4
Step 4 Preparation of (2S,4S)-tert-butyl 2-cyano-4-fluoropyrrolidine-1-carboxylate (2S,4R)-tert-Butyl 2-cyano-4-hydroxypyrrolidine-1-carboxylate 13d (49.7 g, 0.2344 mol) was dissolved in 1130 mL of dichloromethane with stirring under argon atmosphere. The mixture was cooled to -30 °C followed by addition of diethylaminosulfur trifluoride (56.7 g, 0.3516 mol). After stirred for 45 minutes, the reaction mixture was warmed up to -5 °C. Then the mixture was reacted overnight at room temperature. The reaction was monitored by TLC until the disappearance of the starting materials. The reaction mixture was neutralized with saturated aqueous sodium carbonate solution to pH > 7 at such a rate that the reaction temperature was kept below 20 °C. Then ice water and 500 mL of dichloromethane were added. The separated organic phase was washed with 500 mL of saturated aqueous sodium hydrogen sulfate and 500 mL of saturated brine successively, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure in which the concentration temperature was kept below 38 °C to obtain the title compound (2S,4S)-tert-butyl 2-cyano-4-fluoropyrrolidine-1-carboxylate 13e (50 g, yield 100%) as a light yellow solid.
References:
Shanghai Hengrui Pharmaceutical Co. Ltd.;Jiangsu Hansoh Pharmaceutical Co., Ltd. EP2246347, 2010, A1 Location in patent:Page/Page column 39

426844-22-6
22 suppliers
inquiry

426844-76-0
106 suppliers
inquiry
74844-91-0
417 suppliers
$5.00/5g

426844-76-0
106 suppliers
inquiry
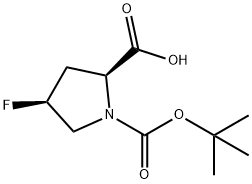
203866-13-1
223 suppliers
$6.00/250mg

426844-76-0
106 suppliers
inquiry
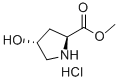
40216-83-9
331 suppliers
$6.00/5g

426844-76-0
106 suppliers
inquiry