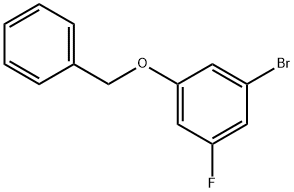
1-(benzyloxy)-3-broMo-5-fluorobenzene synthesis
- Product Name:1-(benzyloxy)-3-broMo-5-fluorobenzene
- CAS Number:130722-44-0
- Molecular formula:C13H10BrFO
- Molecular Weight:281.12
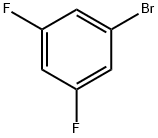
461-96-1
485 suppliers
$6.00/10g
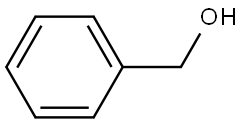
100-51-6
1433 suppliers
$5.00/100g

130722-44-0
36 suppliers
$46.00/1g
Yield:130722-44-0 98%
Reaction Conditions:
Stage #1: benzyl alcoholwith sodium hydride in tetrahydrofuran; for 1 h;
Stage #2: 3,5-difluorobromobenzene in tetrahydrofuran at 20; for 49 h;
Steps:
25.i Example 25; Ph(3-F)(5-OCHF2)-(R)CH(OH)C(O)-Aze-Pab x TFA; (i) 1-Bromo-3-fluoro-5-benzyloxybenzene
Sodium hydride (60% dispersion in oil, 24.0 g, 0.48 mol) was added portionwise to a stirred solution of anhydrous benzyl alcohol (64.5 g, 0.60 mol) in THF (1.0 L). After the mixture was stirred for 1 h, a solution of [1-BROM-3,] 5-difluorobenzene (76.8 g, 0.40 mmol) in THF (100 mL) was added dropwise over a period of 1 h. The reaction was stirred at room temperature for 2 d. Water (400 [ML)] was added and the THF was removed in vacuo. The aqueous layer was extracted with hexane (3 x 150 mL). The combined organic extracts were washed with 2N [NAOH] (2 x 100 mL) then, dried (Na2SO4), filtered and concentrated in vacuo to afford the sub-title compound (110.7 g, 98%) as a light yellow oil that was used without further purification. [RF =] 0.47 (Hex) 'H NMR (300 MHz, [CDC13)] [5] 7.36-7. 41 (m, [5H),] 6.94 (bs, 1H), 6.87 (d, [JH-F] = 8 Hz, [1H),] 6.63 [(D,] [JH-F] = 10 Hz, [IH),] 5.03 (s, 2H).
References:
WO2003/101956,2003,A1 Location in patent:Page 88
433939-27-6
194 suppliers
$5.00/1g

100-39-0
438 suppliers
$10.00/10g

130722-44-0
36 suppliers
$46.00/1g
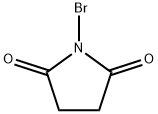
128-08-5
875 suppliers
$5.00/5G

461-96-1
485 suppliers
$6.00/10g
130723-09-0
7 suppliers
inquiry

130722-44-0
36 suppliers
$46.00/1g
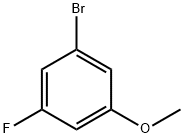
29578-39-0
189 suppliers
$6.00/1g

130722-44-0
36 suppliers
$46.00/1g