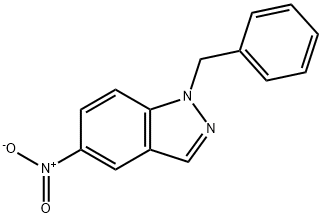
1-BENZYL-5-NITRO-1H-INDAZOLE synthesis
- Product Name:1-BENZYL-5-NITRO-1H-INDAZOLE
- CAS Number:23856-20-4
- Molecular formula:C14H11N3O2
- Molecular Weight:253.26

100-39-0
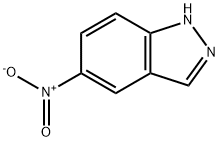
5401-94-5

23856-20-4
To a solution of 5-nitroindazole (10.0 g, 61.3 mmol) in acetonitrile (100 mL) was added potassium carbonate (16.9 g, 122.6 mmol) and benzyl bromide (13.6 g, 79.7 mmol). The reaction mixture was heated at 70 °C with stirring overnight. After completion of the reaction, it was cooled to room temperature, the solid was collected by filtration and washed with dichloromethane. The filtrate was concentrated to dryness and the residue was purified by fast column chromatography using a hexane solution of 17-25% ethyl acetate (v/v) as eluent to give 1-benzyl-5-nitro-1H-indazole (7.0 g, 44%) as a yellow solid. Iron powder (4.03 g, 72.1 mmol) was slowly added to a solution of 1-benzyl-5-nitro-1H-indazole (6.9 g, 27.2 mmol) in acetic acid (200 mL). After stirring at room temperature overnight, the reaction mixture became milky and a white precipitate was formed. The precipitate was removed by filtration and the filtrate was concentrated to about 20 mL. The residue was diluted with water (200 mL) and slowly neutralized with sodium hydroxide. Subsequently, the mixture was extracted with ethyl acetate (5 × 100 mL). The organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated to dryness to give 1-benzyl-1H-imidazol-5-ylamine (5.23 g, 82%) as a brown solid. 1H NMR (DMSO-D6): δ 7.72 (s, 1H), 7.35 (d, J = 8.8 Hz, 1H), 7.24-7.14 (m, 5H), 6.74 (m, 2H), 5.49 (s, 2H), 4.80 (br, 2H). ES-LCMS: RT = 0.93 min; [M + H]+ = 224.2.
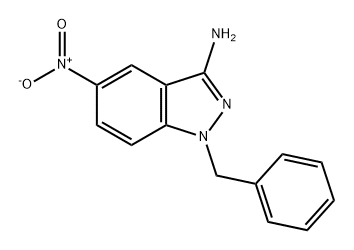
451459-99-7
0 suppliers
inquiry

23856-20-4
54 suppliers
$18.00/250mg
Yield:23856-20-4 90%
Reaction Conditions:
with tert.-butylnitrite in tetrahydrofuran; for 1 h;Reflux;
Steps:
1 General procedure for synthesis of indazole 3
General procedure: A mixture of 3-amino-1-methyl-1H-indazole 2 (3.0 mmol) and tert-butyl nitrite (1.0 mL, 8.1 mmol, 2.7 equiv) in THF (12.0 mL) was heated to reflux for 1 h. The mixture was cooled to rt and then concentrated. H2O (10.0 mL) and EtOAc (20.0 mL) were added to the residue. The organic layer was washed with H2O (10.0 mL), brine (10.0 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The residue was subjected to silica-gel chromatography by using Et2O/hexanes (1:4) as eluent to give the product 3.
References:
Liu, Han-Jun;Hung, Shiang-Fu;Chen, Chuan-Lin;Lin, Mei-Huey [Tetrahedron,2013,vol. 69,# 19,p. 3907 - 3912]

100-39-0
433 suppliers
$10.00/10g

5401-94-5
375 suppliers
$14.00/5g

23856-20-4
54 suppliers
$18.00/250mg

100-39-0
433 suppliers
$10.00/10g

5401-94-5
375 suppliers
$14.00/5g

187668-23-1
3 suppliers
inquiry

23856-20-4
54 suppliers
$18.00/250mg

27996-87-8
179 suppliers
$5.00/1g
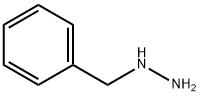
555-96-4
82 suppliers
inquiry

23856-20-4
54 suppliers
$18.00/250mg
100-44-7
658 suppliers
$13.50/250G

5401-94-5
375 suppliers
$14.00/5g

23856-20-4
54 suppliers
$18.00/250mg