
1-Adamantaneacetic acid synthesis
- Product Name:1-Adamantaneacetic acid
- CAS Number:4942-47-6
- Molecular formula:C12H18O2
- Molecular Weight:194.27
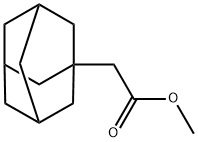
27174-71-6
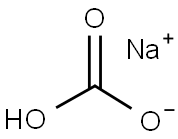
144-55-8

4942-47-6
General procedure for synthesizing 1-adamantylacetic acid from methyl 2-(adamantan-1-yl)acetate and sodium bicarbonate: referring to the preparation of 2-adamantylacetic acid in Example 10, 2.53 g of sodium hydroxide was dissolved in 433 mL of methanol, and 1,125 mL of methanol solution containing 150 g (0.72 mol) of methyl 2-adamantylacetic acid was added, and the reaction was stirred at room temperature for 6 hours at room temperature with stirring. Upon completion of the reaction, the reaction mixture was concentrated under vacuum. To the concentrated residue was added aqueous sodium bicarbonate solution and the mixture was washed with ether. Subsequently, the aqueous layer was adjusted to pH 1 with 12N hydrochloric acid and extracted with ethyl acetate. The combined extracts were dried with anhydrous sodium sulfate. After filtration to remove the desiccant, the filtrate was concentrated in vacuum to give the target product 1-adamantaneacetic acid. Yield: 129.39 g, 93% yield.

27174-71-6
38 suppliers
$75.00/500mg

144-55-8
1373 suppliers
$10.00/25g

4942-47-6
301 suppliers
$6.00/1g
Yield:4942-47-6 93%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in methanol
Steps:
R.10 Referential Example 10
Referential Example 10 Production of 2-adamantyl acetic acid: 2.5N sodium hydroxide 433 ml was added to methanol 1125 ml solution of 2-adamantyl acetic acid methyl ester 150 g (0.72 mole) and stirred at room temperature for 6 hours. The reaction mixture was concentrated in vacuo, and aqueous sodium bicarbonate was added to the residue and the mixture was washed with ether. Aqueous layer was adjusted to pH I by adding 12N hydrochloric acid and extracted with ethylacetate. The extract was dried by anhydrous sodium sulfate. After removing the drying agent by filtration, the filtrate was concentrated in vacuo to obtain the product. Yield: 129.39 g (Yield 93%)
References:
Asahi Kasei Kogyo Kabushiki Kaisha US5658923, 1997, A
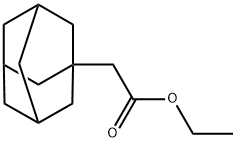
15782-66-8
29 suppliers
$95.00/500mg

4942-47-6
301 suppliers
$6.00/1g
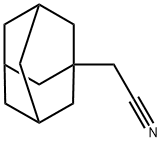
16269-13-9
61 suppliers
$80.00/2 g

4942-47-6
301 suppliers
$6.00/1g

27174-71-6
38 suppliers
$75.00/500mg

4942-47-6
301 suppliers
$6.00/1g

91233-19-1
1 suppliers
inquiry

4942-47-6
301 suppliers
$6.00/1g