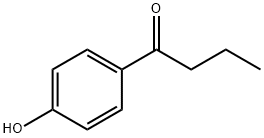
1-(4-Hydroxyphenyl)-1-butanone synthesis
- Product Name:1-(4-Hydroxyphenyl)-1-butanone
- CAS Number:1009-11-6
- Molecular formula:C10H12O2
- Molecular Weight:164.2

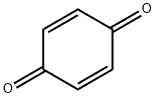
141-75-3
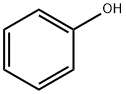
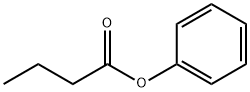
108-95-2

1009-11-6
General method: phenol (0.28 mmol) and n-butyryl chloride (0.28 mmol) were dissolved in trifluoromethanesulfonic acid (TfOH, 3 mL) at 0°C. The reaction mixture was gradually warmed up to room temperature and the reaction was carried out for the time shown in Table 2. Upon completion of the reaction, the mixture was poured into a mixture of cold water and ethyl acetate. The organic layer was separated and washed sequentially with 1 M hydrochloric acid, saturated sodium bicarbonate solution and saturated sodium chloride solution. The organic layer was dried with anhydrous magnesium sulfate, filtered and the filtrate concentrated. The residue was purified by silica gel column chromatography to give 4'-hydroxybutyrophenone. The spectral data of all the products were in agreement with those reported in the literature.

141-75-3
359 suppliers
$12.32/25G

108-95-2
771 suppliers
$14.00/25g

1009-11-6
211 suppliers
$11.00/5g
Yield:1009-11-6 98%
Reaction Conditions:
with trifluorormethanesulfonic acid at 0 - 20; for 1 h;regiospecific reaction;Friedel-Crafts acylation;
Steps:
4.3. General procedure of Friedel-Crafts acylation of phenols in TfOH
General procedure: Phenol (0.28 mmol) and acyl chloride (0.28 mmol) were dissolved in TfOH (3 ml) at 0 °C. The reaction mixture was warmed to room temperature for appropriate time in Table 2, then poured into cold water and ethyl acetate. The organic layer was washed with 1 M HCl, saturated NaHCO3, and saturated NaCl, and dried over MgSO4, then filtrated. The filtrate was concentrated and the residue was subjected silica column chromatography to afford acylated products. All spectral data were identical with the literatures.27
References:
Murashige, Ryo;Hayashi, Yuka;Ohmori, Syo;Torii, Ayuko;Aizu, Yoko;Muto, Yasuyuki;Murai, Yuta;Oda, Yuji;Hashimoto, Makoto [Tetrahedron,2011,vol. 67,# 3,p. 641 - 649] Location in patent:experimental part

627-19-0
114 suppliers
$10.00/1g

108-95-2
771 suppliers
$14.00/25g

1009-11-6
211 suppliers
$11.00/5g
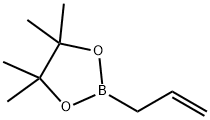
72824-04-5
213 suppliers
$6.00/1g
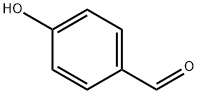
123-08-0
961 suppliers
$5.00/10g

1009-11-6
211 suppliers
$11.00/5g