1-(4-AMINO-3-BROMO-PHENYL)-ETHANONE synthesis
- Product Name:1-(4-AMINO-3-BROMO-PHENYL)-ETHANONE
- CAS Number:56759-32-1
- Molecular formula:C8H8BrNO
- Molecular Weight:214.06
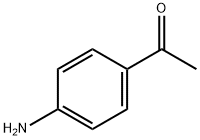
99-92-3

56759-32-1
The general procedure for the synthesis of 4''-amino-3''-bromoacetophenone from 4-aminoacetophenone was as follows: 1-(4-aminophenyl)ethanone (10.0 g, 74.0 mmol) was dissolved in acetonitrile (50 mL), and an acetonitrile solution of N-bromosuccinimide (13.8 g, 77.7 mmol) was slowly added dropwise (30 mL) under ice-bath cooling conditions. After the dropwise addition, the reaction mixture was stirred at room temperature for 20 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue obtained was dissolved in ethyl acetate. The organic layer was washed sequentially with saturated aqueous sodium bicarbonate and saturated saline and dried with anhydrous sodium sulfate. Finally, the solvent was evaporated under reduced pressure to afford the target product 4''-amino-3''-bromoacetophenone as a yellow powder (15.8 g, 100% yield). The structure of the product was confirmed by 1H-NMR (CDCl3, 400 MHz): δ 2.49 (3H, s), 4.52-4.70 (2H, br), 6.73 (1H, d, J = 8.5 Hz), 7.73 (1H, dd, J = 8.5, 1.9 Hz), 8.05 (1H, d, J = 1.9 Hz).

99-92-3
527 suppliers
$6.00/10g

56759-32-1
92 suppliers
inquiry
Yield: 100%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 20 h;Cooling with ice;
Steps:
23a (23a) 1-(4-amino-3-bromophenyl)ethanone
Example 23 4-acetyl-2-{4-[2-(tetrahydropyran-4-yloxy)ethoxy]phenylamino}benzonitrile (23a) 1-(4-amino-3-bromophenyl)ethanone 1-(4-Aminophenyl)ethanone (10.0 g, 74.0 mmol) was dissolved in acetonitrile (50 mL) and, under ice-cooling, a solution (30 mL) of N-bromosuccinimide (13.8 g, 77.7 mmol) in acetonitrile was added dropwise, and the mixture was stirred at room temperature for 20 hr. The solvent of the reaction mixture was evaporated under reduced pressure, and the obtained residue was dissolved in ethyl acetate. The organic layer was washed successively with saturated aqueous sodium hydrogen carbonate and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the title object compound as a yellow powder (15.8 g, yield 100%). 1H-NMR (CDCl3, 400 MHz) δ: 2.49 (3H, s), 4.52-4.70 (2H, br), 6.73 (1H, d, J=8.5 Hz), 7.73 (1H, dd, J=8.5, 1.9 Hz), 8.05 (1H, d, J=1.9 Hz).
References:
Kyoto Pharmaceutical Industries, Ltd.;SHIRAHASE, Hiroaki;TAKAHASHI, Kenji;SHOJI, Yoshimichi;TAKEDA, Shigemitsu US2016/207883, 2016, A1 Location in patent:Paragraph 0644-0646
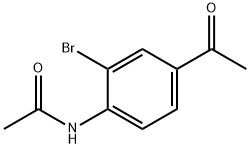
101209-08-9
70 suppliers
$66.00/5g

56759-32-1
92 suppliers
inquiry

99-92-3
527 suppliers
$6.00/10g
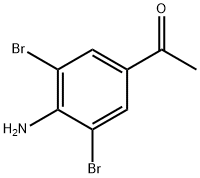
22589-50-0
39 suppliers
$165.46/0.5g

56759-32-1
92 suppliers
inquiry
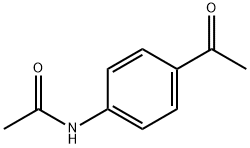
2719-21-3
113 suppliers
$14.00/1g

56759-32-1
92 suppliers
inquiry