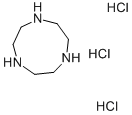
1,4,7-Triazacyclononane trihydrochloride synthesis
- Product Name:1,4,7-Triazacyclononane trihydrochloride
- CAS Number:58966-93-1
- Molecular formula:C6H18Cl3N3
- Molecular Weight:238.59
A round bottom flask (1 L) was charged with 18M H2SO4 (450 mL) and 1,4,7-tris(p-toluenesulfonyl)-1,4,7-triazacyclononane (4) (138.8 g, 0.234 mol) added in small portions (approximately 10 g every 5 min). The mixture was heated with stirring in a heat block for 3 days at 120°C. The resulting black solution was cooled to room temperature and added dropwise using a dropping funnel to a vigorously stirred mixture of cold absolute EtOH/Et2O (1.5 L/900 mL) cooled in an ice bath. An overhead stirrer was used to ensure efficient stirring. A sticky hygroscopic brown/grey precipitate formed, which was isolated quickly by vacuum filtration and immediately dissolved in de–ionised H2O (1 L). The mixture was heated for 2 h at 60°C. The mixture was then cooled to room temperature, filtered through Celite and the resulting solution concentrated to 250 mL under reduced pressure at 65°C. Conc. HCl (200 mL) was added followed by absolute EtOH until the solution became cloudy. The mixture was stored at 4°C overnight to promote precipitation. The white precipitate was collected by filtration and washed with ice cold absolute EtOH (3 x 50 mL), followed by Et2O (2 x 50 mL) to yield tacn.3HCl (5) as a white crystalline powder. Yield 44.9 g (80%).
mp. 268.1–270.0°C (Lit.4 mp. 280–281°C). 1H NMR (300 MHz, D2O) δH 3.48 (12H, s, CH2). 13C NMR (75 MHz, D2O) δC 43.1 (CH2). ESI–MS m/z [M + H]+ 130.1. The 1H and 13C NMR spectral data were consistent with literature data.
Using iSUSTAIN(TM) to validate the chemical attributes of different approaches to the synthesis of tacn and bridged bis(tacn) ligands
![1,4,7-tris[(4-Methylphenyl)sulfonyl]-1,4,7-triazonane](/CAS/GIF/52667-89-7.gif)
52667-89-7
62 suppliers
$14.00/100mg

58966-93-1
181 suppliers
$10.00/250mg
Yield:58966-93-1 99%
Reaction Conditions:
Stage #1: N,N',N-tris(p-toluenesulfonyl)-1,4,7-triazacyclononanewith sulfuric acid at 100; for 72 h;Inert atmosphere;
Stage #2: with sodium hydroxide in water;Inert atmosphere;
Stage #3: with hydrogenchloride in water; pH=1;
References:
Eitel, Simon H.;Bauer, Matthias;Schweinfurth, David;Deibel, Naina;Sarkar, Biprajit;Kelm, Harald;Krueger, Hans-Joerg;Frey, Wolfgang;Peters, Rene [Journal of the American Chemical Society,2012,vol. 134,# 10,p. 4683 - 4693] Location in patent:supporting information; experimental part
![1H-1,4,7-Triazonine, octahydro-1,4-bis[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/95388-08-2.gif)
95388-08-2
0 suppliers
inquiry

58966-93-1
181 suppliers
$10.00/250mg

106-93-4
405 suppliers
$15.00/5g
111-40-0
520 suppliers
$13.44/25ML

58966-93-1
181 suppliers
$10.00/250mg