
1-(2-Chloroethyl)pyrrolidine Hydrochloride synthesis
- Product Name:1-(2-Chloroethyl)pyrrolidine Hydrochloride
- CAS Number:7250-67-1
- Molecular formula:C6H13Cl2N
- Molecular Weight:170.08
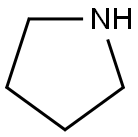
123-75-1

107-07-3

7250-67-1
The general procedure for the synthesis of N-(2-chloroethyl)pyrrolidine hydrochloride from tetrandrine and 2-chloroethanol was as follows: 165 mL (2 mol) of tetrandrine, 67 mL (1 mol) of 2-chloroethanol, and 200 mL of toluene were added sequentially to a reaction flask. The mixture was heated to reflux temperature and kept reacting for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and the solid product was isolated. The solid was collected by diafiltration and the filter cake was washed with 20 mL of toluene. The temperature of the filtrate was controlled at about 75 °C and 200 mL of thionyl chloride was slowly added dropwise. After the dropwise addition was completed, the reaction was continued at reflux for 2 hours. At the end of the reaction, the mixture was cooled to room temperature and subsequently concentrated to dryness under reduced pressure. The resulting residue was recrystallized from anhydrous ethanol to give 105 g of white solid product in 62.3% yield. The melting point of the product was 198-203 °C.
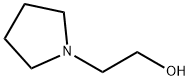
2955-88-6
267 suppliers
$8.00/5g

7250-67-1
261 suppliers
$10.00/5g
Yield:7250-67-1 46%
Reaction Conditions:
with thionyl chloride for 1 h;Heating / reflux;
Steps:
KK
2- pyrrolidin-1-ylethanol (6.24 g, 0.05 mol) was stirred while cautiously adding thionyl chloride (40 ml_, 0.54 mol). The stirred mixture was refluxed for 1 hour under nitrogen atmosphere, then concentrated in vacuo. The residue was triturated with diethyl ether to give a solid, and the liquid decanted; this process was repeated several times, until the decantate was almost colorless. The flask containing solid was evaporated to give a free- flowing, cocoa-colored solid (4.21 g, 46%). 1H-NMR (CD2 Cl2): δ 13.1 (br s, 1 H), 3.97 (t, 3 H), 3.7 (m, 2 H), 3.33 (dd, 2 H), 2.81 (m, 2 H), 2.14 - 1.95 (m, 3 H).
References:
BAYER PHARMACEUTICALS CORPORATION WO2007/56170, 2007, A2 Location in patent:Page/Page column 108